Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis
- PMID: 22017344
- PMCID: PMC3288433
- DOI: 10.1111/j.1530-0277.2011.01660.x
Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis
Abstract
Background: Chronic ethanol consumption increases the risk of hepatic cirrhosis and hepatocellular carcinoma (HCC). While sex differences exist in susceptibility to ethanol-induced liver damage/HCC development, little is known about the effects of ethanol on tumor progression.
Methods: Neonatal male and female mice were initiated with a single dose of diethylnitrosamine (DEN). Sixteen or 40 weeks later, animals were placed on a 10/20% (v/v) ethanol-drinking water (EtOH-DW; alternate days) regime for 8 weeks. At study end, liver tissue and serum were analyzed for liver pathology/function and cytokine expression.
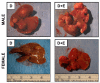
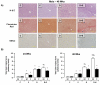
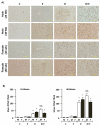
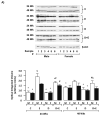
Results: DEN reproducibly induced hepatic foci/tumors in male and female mice. Ethanol diminished hepatic function and increased liver damage, but ethanol alone did not induce hepatic foci/HCC formation. In DEN-initiated EtOH-DW animals, ethanol significantly increased tumor incidence and burden, but only in male mice. Male and female mice (±DEN) demonstrated comparable blood alcohol content at necropsy, yet increased hepatic damage and diminished hepatic function/antioxidant capacity were significantly greater in males. Analysis of liver mRNA for Th1, Th2, or T-regulatory factors demonstrated significantly elevated SMAD3 in male compared to female mice in response to EtOH, DEN initiation, and DEN + EtOH-DW.
Conclusions: These data demonstrate male mice are more susceptible to HCC incidence and progression in the setting of chronic ethanol feeding than females. Differences in markers of hepatic immune response in male mice suggest that increased TGFβ-SMAD3 signaling may enhance promotion in this model of HCC progression, effects modulated by chronic ethanol feeding.
Copyright © 2011 by the Research Society on Alcoholism.
Figures




Comment in
-
Commentary with regard to the role of alcohol and cofactors in the development of hepatocellular carcinoma.Alcohol Clin Exp Res. 2012 Apr;36(4):564-5. doi: 10.1111/j.1530-0277.2012.01780.x. Epub 2012 Mar 13. Alcohol Clin Exp Res. 2012. PMID: 22414007
References
-
- Albano E, French SW, Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci. 1999;4:D533–40. - PubMed
-
- Castañeda F, Kinne RKH. Cytotoxicity of millimolar concentrations of ethanol on HepG2 human tumor cell line compared to normal rat hepatocytes in vitro. Journal of Cancer Research and Clinical Oncology. 2000;126(9):503–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

