Physiologically relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds
- PMID: 22017359
- PMCID: PMC3237771
- DOI: 10.1021/bm201328k
Physiologically relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds
Abstract
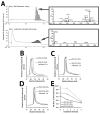
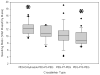
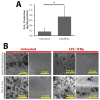
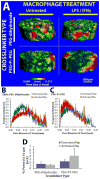
Chronic inflammation-mediated oxidative stress is a common mechanism of implant rejection and failure. Therefore, polymer scaffolds that can degrade slowly in response to this environment may provide a viable platform for implant site-specific, sustained release of immunomodulatory agents over a long time period. In this work, proline oligomers of varying lengths (P(n)) were synthesized and exposed to oxidative environments, and their accelerated degradation under oxidative conditions was verified via high performance liquid chromatography and gel permeation chromatography. Next, diblock copolymers of poly(ethylene glycol) (PEG) and poly(ε-caprolactone) (PCL) were carboxylated to form 100 kDa terpolymers of 4%PEG-86%PCL-10%cPCL (cPCL = poly(carboxyl-ε-caprolactone); i% indicates molar ratio). The polymers were then cross-linked with biaminated PEG-P(n)-PEG chains, where P(n) indicates the length of the proline oligomer flanked by PEG chains. Salt-leaching of the polymeric matrices created scaffolds of macroporous and microporous architecture, as observed by scanning electron microscopy. The degradation of scaffolds was accelerated under oxidative conditions, as evidenced by mass loss and differential scanning calorimetry measurements. Immortalized murine bone-marrow-derived macrophages were then seeded on the scaffolds and activated through the addition of γ-interferon and lipopolysaccharide throughout the 9-day study period. This treatment promoted the release of H(2)O(2) by the macrophages and the degradation of proline-containing scaffolds compared to the control scaffolds. The accelerated degradation was evidenced by increased scaffold porosity, as visualized through scanning electron microscopy and X-ray microtomography imaging. The current study provides insight into the development of scaffolds that respond to oxidative environments through gradual degradation for the controlled release of therapeutics targeted to diseases that feature chronic inflammation and oxidative stress.
Figures
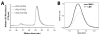


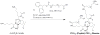

References
-
- Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS letters. 2000;486:10–3. - PubMed
-
- Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–90. - PubMed
-
- Cammas S, Suzuki K, Sone C, Sakurai Y, Kataoka K, Okano T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. Journal of Controlled Release. 1997;48:157–164.
-
- Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discovery Today. 2002;7:569–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

