The role of EGF receptor ubiquitination in regulating its intracellular traffic
- PMID: 22017370
- PMCID: PMC3261333
- DOI: 10.1111/j.1600-0854.2011.01305.x
The role of EGF receptor ubiquitination in regulating its intracellular traffic
Abstract
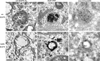
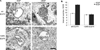
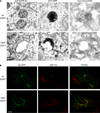
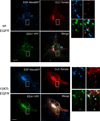
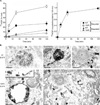
Progression of activated EGF receptor (EGFR) through the endocytic pathway regulates EGFR signaling. Here we show that a non-ubiquitinated EGFR mutant, unable to bind the endosomal-sorting complex required for transport (ESCRT) component, Hrs, is not efficiently targeted onto intraluminal vesicles (ILVs) of multivesicular endosomes/bodies (MVBs). Moreover, ubiquitination and ESCRT engagement of activated EGFR are required for EGF-stimulated ILV formation. Non-ubiquitinated EGFRs enter clathrin-coated tubules emanating from MVBs and show enhanced recycling to the plasma membrane, compared to wild-type EGFR.
© 2011 John Wiley & Sons A/S.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

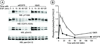
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

