Enzymatic chemistry of cyclopropane, epoxide, and aziridine biosynthesis
- PMID: 22017381
- PMCID: PMC3288687
- DOI: 10.1021/cr200073d
Enzymatic chemistry of cyclopropane, epoxide, and aziridine biosynthesis
Figures
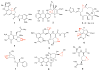

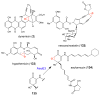

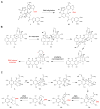



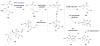
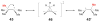






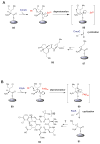





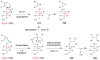
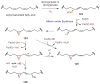

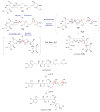
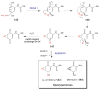











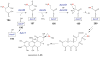

References
-
- Steglich W, Fugmann B, Lang-Fugmann S, editors. Römpp Encyclopedia Natural Products. Georg Thieme Verlag; Stuttgart, Germany: 2000.
-
- Takahashi I, Takahashi K, Ichinura M, Morimoto M, Asano K, Kawamoto I, Tomita F, Nakano H. J Antibiot. 1988;41:1915. - PubMed
-
- Konichi M, Ohkuma H, Matusumoto K, Tsuno T, Kamei H, Miyaki T, Oki T, Kawaguchi H, Van Duyne GD, Clardy J. J Antibiot. 1989;42:1449. - PubMed
-
- Hofle G, Bedorf N, Steinmetz H, Schomburg D, Gerth K, Reichenbach H. Angew Chem Int Ed. 1996;35:1567.
-
- Tomasz M, Palom Y. Pharmacol Therap. 1997;76:73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

