The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation
- PMID: 22017438
- PMCID: PMC3381650
- DOI: 10.1111/j.1600-065X.2011.01064.x
The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation
Abstract
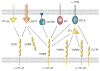
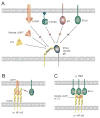
The tumor necrosis factor (TNF) receptor superfamily member herpesvirus entry mediator (HVEM) (TNFRSF14) regulates T-cell immune responses by activating both inflammatory and inhibitory signaling pathways. HVEM acts as both a receptor for the canonical TNF-related ligands, LIGHT [lymphotoxin-like, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for HVEM, a receptor expressed on T lymphocytes] and lymphotoxin-α, and as a ligand for the immunoglobulin superfamily proteins BTLA (B and T lymphocyte attenuator) and CD160, a feature distinguishing HVEM from other immune regulatory molecules. The ability of HVEM to interact with multiple ligands in distinct configurations creates a functionally diverse set of intrinsic and bidirectional signaling pathways that control both inflammatory and inhibitory responses. The HVEM system is integrated into the larger LTβR and TNFR network through extensive shared ligand and receptor usage. Experimental mouse models and human diseases indicate that dysregulation of HVEM network may contribute to autoimmune pathogenesis, making it an attractive target for drug intervention.
© 2011 John Wiley & Sons A/S.
Figures

References
-
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. - PubMed
-
- Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344:17–24. - PubMed
-
- Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. - PubMed
-
- Ware CF. Network communications: Lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

