Enhanced athletic performance on multisite AAV-IGF1 gene transfer coincides with massive modification of the muscle proteome
- PMID: 22017471
- PMCID: PMC3277730
- DOI: 10.1089/hum.2011.157
Enhanced athletic performance on multisite AAV-IGF1 gene transfer coincides with massive modification of the muscle proteome
Abstract
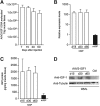
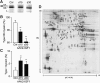
Progress in gene therapy has hinted at the potential misuse of gene transfer in sports to achieve better athletic performance, while escaping from traditional doping detection methods. Suitable animal models are therefore required in order to better define the potential effects and risks of gene doping. Here we describe a mouse model of gene doping based on adeno-associated virus (AAV)-mediated delivery of the insulin-like growth factor-I (IGF-I) cDNA to multiple muscles. This treatment determined marked muscle hypertrophy, neovascularization, and fast-to-slow fiber type transition, similar to endurance exercise. In functional terms, treated mice showed impressive endurance gain, as determined by an exhaustive swimming test. The proteomic profile of the transduced muscles at 15 and 30 days after gene delivery revealed induction of key proteins controlling energy metabolism. At the earlier time point, enzymes controlling glycogen mobilization and anaerobic glycolysis were induced, whereas they were later replaced by proteins required for aerobic metabolism, including enzymes related to the Krebs cycle and oxidative phosphorylation. These modifications coincided with the induction of several structural and contractile proteins, in agreement with the observed histological and functional changes. Collectively, these results give important insights into the biological response of muscles to continuous IGF-I expression in vivo and warn against the potential misuse of AAV-IGF1 as a doping agent.
Figures





Comment in
-
A new approach to winning: insulin-like growth factor-I signaling in competitive sport.Hum Gene Ther. 2012 Feb;23(2):139-40. doi: 10.1089/hum.2012.2500. Hum Gene Ther. 2012. PMID: 22324821 No abstract available.
References
-
- Alban A. David S.O. Bjorkesten L., et al. A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. - PubMed
-
- Arsic N. Zentilin L. Zacchigna S., et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol. Ther. 2003;7:450–459. - PubMed
-
- Benito M. Valverde A.M. Lorenzo M. IGF-I: A mitogen also involved in differentiation processes in mammalian cells. Int. J. Biochem. Cell Biol. 1996;28:499–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
