Innate and guided C-H functionalization logic
- PMID: 22017496
- PMCID: PMC3288638
- DOI: 10.1021/ar200194b
Innate and guided C-H functionalization logic
Abstract
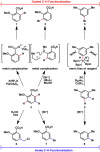
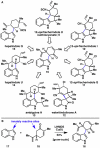
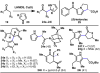
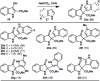
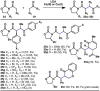
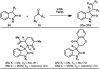
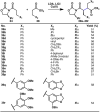
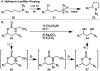
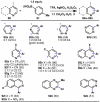
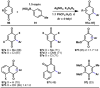
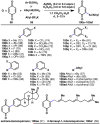
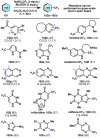
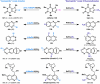
The combustion of organic matter is perhaps the oldest and most common chemical transformation utilized by mankind. The generation of a C-O bond at the expense of a C-H bond during this process may be considered the most basic form of C-H functionalization. This illustrates the extreme generality of the term "C-H functionalization", because it can describe the conversion of literally any C-H bond into a C-X bond (X being anything except H). Therefore, it may be of use to distinguish between what, in our view, are two distinct categories of C-H functionalization logic: "guided" and "innate". Guided C-H functionalizations, as the name implies, are guided by external reagents or directing groups (covalently or fleetingly bound) to install new functional groups at the expense of specifically targeted C-H bonds. Conversely, innate C-H functionalizations may be broadly defined as reactions that exchange C-H bonds for new functional groups based solely on natural reactivity patterns in the absence of other directing forces. Two substrates that illustrate this distinction are dihydrojunenol and isonicotinic acid. The C-H functionalization processes of hydroxylation or arylation, respectively, can take place at multiple locations on each molecule. Innate functionalizations lead to substitution patterns that are dictated by the inherent bias (steric or electronic) of the substrate undergoing C-H cleavage, whereas guided functionalizations lead to substitution patterns that are controlled by external directing forces such as metal complexation or steric bias of the reagent. Although the distinction between guided and innate C-H functionalizations may not always be clear in cases that do not fit neatly into a single category, it is a useful convention to consider when analyzing reactivity patterns and strategies for synthesis. We must emphasize that although a completely rigorous distinction between guided and innate C-H functionalization may not be practical, we have nonetheless found it to be a useful tool at the planning stage of synthesis. In this Account, we trace our own studies in the area of C-H functionalization in synthesis through the lens of "guided" and "innate" descriptors. We show how harnessing innate reactivity can be beneficial for achieving unique bond constructions between heterocycles and carbonyl compounds, enabling rapid and scalable total syntheses. Guided and innate functionalizations were used synergistically to create an entire family of terpenes in a controlled fashion. We continue with a discussion of the synthesis of complex alkaloids with high nitrogen content, which required the invention of a uniquely chemoselective innate C-H functionalization protocol. These findings led us to develop a series of innate C-H functionalization reactions for forging C-C bonds of interest to the largest body of practicing organic chemists: medicinal chemists. Strategic use of C-H functionalization logic can have a dramatically positive effect on the efficiency of synthesis, whether guided or innate.
Figures







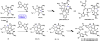


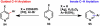


References
-
- Gutekunst WR, Baran PS. C-H Functionalization Logic in Total Synthesis. Chem. Soc. Rev. 2011;40:1976–1991. - PubMed
-
- Nguyen T-H, Chau NTT, Castanet A-S, Nguyen KPP, Mortier J. First General, Direct, and Regioselective Synthesis of Substituted Methoxybenzoic Acids by Ortho Metalation. J. Org. Chem. 2007;72:3419–3429. - PubMed
- Wang D-H, Mei T-S, Yu J-Q. Versatile Pd(II)-Catalyzed C−H Activation/Aryl−Aryl Coupling of Benzoic and Phenyl Acetic Acids. J. Am. Chem. Soc. 2008;130:17676–17677. - PMC - PubMed
- Murphy JM, Liao X, Hartwig JF. Meta Halogenation of 1,3-Disubstituted Arenes Via Iridium-Catalyzed Arene Borylation. J. Am. Chem. Soc. 2007;129:15434–15435. - PubMed
-
- Onda K, Shiraki R, Ogiyama T, Yokoyama K, Momose K, Katayama N, Orita M, Yamaguchi T, Furutani M, Hamada N, Takeuchi M, Okada M, Ohta M, Tsukamoto S-I. Design, Synthesis, and Pharmacological Evaluation of N-Bicyclo-5-Chloro-1H-Indole-2-Carboxamide Derivatives as Potent Glycogen Phosphorylase Inhibitors. Bioorg. Med. Chem. 2008;16:10001–10012. - PubMed
- Leonard F, Wajngurt A, Tschannen W, Block FB. Unnatural Amino Acids. I. 3-Carboxytyrosine Derivatives. J. Med. Chem. 1965;8:812–815.
- Smith MB, March J. March’s Advanced Organic Chemistry. John Wiley & Sons; Hoboken: 2007.
-
-
C-H Activation. In Topics in Current Chemistry; Yu J-Q, Shi Z, editors. Vol. 292. Springer Berlin; Heidelberg: 2010. p. 380.
-
-
- Sundberg RJ. Indoles. Academic Press; San Diego: 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

