Expression patterns of intestinal calcium transport factors and ex-vivo absorption of calcium in horses
- PMID: 22017756
- PMCID: PMC3221622
- DOI: 10.1186/1746-6148-7-65
Expression patterns of intestinal calcium transport factors and ex-vivo absorption of calcium in horses
Abstract
Background: In many species, the small intestine is the major site of calcium (Ca(2+)) absorption. The horse differs considerably from most other species with regard to the physiology of its Ca(2+) metabolism and digestion. Thus, this study was performed to get more information about the transcellular Ca(2+) absorption in the horse.Two mechanisms of intestinal Ca(2+) absorption are described: the passive paracellular pathway and the active, vitamin D-dependent transcellular pathway. The latter involves the following elements: vitamin D receptors (VDR), transient receptor potential vanilloid channel members 5 and 6 (TRPV5/6), calbindin-D9k (CB), the Na/Ca exchanger (NCX1) and the plasma membrane Ca-ATPase (PMCA). The aim of the present study was to investigate the protein and mRNA expression patterns of VDR, CB and TRPV6 and the ex-vivo Ca(2+) absorption in horses, assessed by qualitative and quantitative RT-PCR, western blot, immunohistochemistry and the Ussing chamber technique.
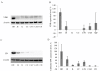
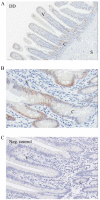
Results: Highest CB and TRPV6 mRNA levels were detected in the duodenum as compared to the middle parts of the jejunum and ileum and several sites of the large intestine. VDR mRNA levels did not change significantly throughout the intestine. TRPV5 mRNA was not detectable in the horse intestine. The highest VDR and CB protein levels were measured in the duodenum. Ussing chamber studies revealed ex-vivo Ca(2+) absorption only in the duodenum, but not in cecum and specific sites of the colon.
Conclusion: The present findings suggest that TRPV6, CB and VDR may be involved in active intestinal Ca(2+) absorption in horses, as described for other mammals. TRPV5 may not play a major role in this process. Furthermore, the expression patterns of these Ca(2+) transport elements and the results of the Ussing chamber procedure indicate that a significant part of active intestinal Ca(2+) absorption occurs in the duodenum in this species.
Figures




Similar articles
-
Cloning, comparative sequence analysis and mRNA expression of calcium-transporting genes in horses.Gen Comp Endocrinol. 2010 May 15;167(1):6-10. doi: 10.1016/j.ygcen.2010.02.022. Epub 2010 Mar 11. Gen Comp Endocrinol. 2010. PMID: 20226785
-
Tissue-specific expression of the calcium transporter genes TRPV5, TRPV6, NCX1, and PMCA1b in the duodenum, kidney and heart of Equus caballus.J Vet Med Sci. 2011 Nov;73(11):1437-44. doi: 10.1292/jvms.11-0141. Epub 2011 Jul 7. J Vet Med Sci. 2011. PMID: 21737966
-
Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and -D28k knockout mice.Exp Physiol. 2009 Jan;94(1):138-51. doi: 10.1113/expphysiol.2008.044339. Epub 2008 Oct 17. Exp Physiol. 2009. PMID: 18931045
-
Intestinal calcium absorption: Molecular vitamin D mediated mechanisms.J Cell Biochem. 2003 Feb 1;88(2):332-9. doi: 10.1002/jcb.10360. J Cell Biochem. 2003. PMID: 12520535 Review.
-
Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6.Nutr Rev. 2011 Jul;69(7):347-70. doi: 10.1111/j.1753-4887.2011.00395.x. Epub 2011 Jun 3. Nutr Rev. 2011. PMID: 21729089 Review.
Cited by
-
Evaluation and Comparison of Vitamin D Responsive Gene Expression in Ovine, Canine and Equine Kidney.PLoS One. 2016 Sep 15;11(9):e0162598. doi: 10.1371/journal.pone.0162598. eCollection 2016. PLoS One. 2016. PMID: 27632366 Free PMC article.
-
TRPV6 and Calbindin-D9k-expression and localization in the bovine uterus and placenta during pregnancy.Reprod Biol Endocrinol. 2012 Aug 29;10:66. doi: 10.1186/1477-7827-10-66. Reprod Biol Endocrinol. 2012. PMID: 22931437 Free PMC article.
References
-
- Bronner F, Pansu D, Stein WD. An analysis of intestinal calcium transport across the rat intestine. Am J Physiol. 1986;250(5 Pt 1):G561–569. - PubMed
-
- van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ. Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G78–85. - PubMed
-
- Bronner F, Pansu D. Nutritional aspects of calcium absorption. J Nutr. 1999;129(1):9–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

