Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α
- PMID: 22017873
- PMCID: PMC3319689
- DOI: 10.1016/j.molcel.2011.07.037
Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α
Abstract
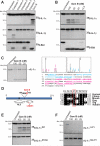
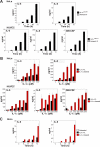
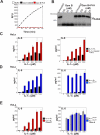
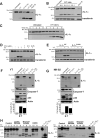
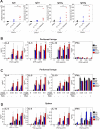
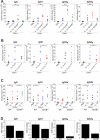
Granzyme B is a cytotoxic lymphocyte-derived protease that plays a central role in promoting apoptosis of virus-infected target cells, through direct proteolysis and activation of constituents of the cell death machinery. However, previous studies have also implicated granzymes A and B in the production of proinflammatory cytokines, via a mechanism that remains undefined. Here we show that IL-1α is a substrate for granzyme B and that proteolysis potently enhanced the biological activity of this cytokine in vitro as well as in vivo. Consistent with this, compared with full-length IL-1α, granzyme B-processed IL-1α exhibited more potent activity as an immunoadjuvant in vivo. Furthermore, proteolysis of IL-1α within the same region, by proteases such as calpain and elastase, was also found to enhance its biological potency. Thus, IL-1α processing by multiple immune-related proteases, including granzyme B, acts as a switch to enhance the proinflammatory properties of this cytokine.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Afonina I, Cullen SP, Martin SJ. Cytotoxic and non cytotoxic roles of the CTL/NK proteases granzyme B. Immunological Reviews. 2010;235:105–116. - PubMed
-
- Bertelsen M, Sanfridson A. TAB1 modulates IL-1alpha mediated cytokine secretion but is dispensable for TAK1 activation. Cell Signal. 2007;19:646–57. - PubMed
-
- Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, Wignall J, Conlon PJ, Cosman D, Hopp TP, et al. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 1988;263:9437–42. - PubMed
-
- Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J. Biol. Chem. 1991;266:12162–7. - PubMed
-
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

