A cortical substrate for memory-guided orienting in the rat
- PMID: 22017991
- PMCID: PMC3212026
- DOI: 10.1016/j.neuron.2011.07.010
A cortical substrate for memory-guided orienting in the rat
Abstract
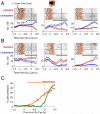
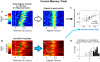
Anatomical, stimulation, and lesion data have suggested a homology between the rat frontal orienting fields (FOF) (centered at +2 AP, ±1.3 ML mm from Bregma) and primate frontal cortices such as the frontal or supplementary eye fields. We investigated the functional role of the FOF using rats trained to perform a memory-guided orienting task, in which there was a delay period between the end of a sensory stimulus instructing orienting direction and the time of the allowed motor response. Unilateral inactivation of the FOF resulted in impaired contralateral responses. Extracellular recordings of single units revealed that 37% of FOF neurons had delay period firing rates that predicted the direction of the rats' later orienting motion. Our data provide the first electrophysiological and pharmacological evidence supporting the existence in the rat, as in the primate, of a frontal cortical area involved in the preparation and/or planning of orienting responses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

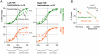



Comment in
-
Movement, confusion, and orienting in frontal cortices.Neuron. 2011 Oct 20;72(2):193-6. doi: 10.1016/j.neuron.2011.10.002. Neuron. 2011. PMID: 22017982
References
-
- Brecht M, Krauss A, Muhammad S, Sinai-Esfahani L, Bellanca S, Margrie TW. Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells. J Comp Neurol. 2004;479:360–373. - PubMed
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. - PubMed
-
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

