Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication
- PMID: 22018238
- PMCID: PMC3201796
- DOI: 10.1016/j.chom.2011.08.015
Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication
Abstract
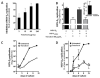
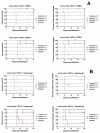
The HIV reverse-transcriptase inhibitor, tenofovir, was recently formulated into a vaginal gel for use as a microbicide. In human trials, a 1% tenofovir gel inhibited HIV sexual transmission by 39% and, surprisingly, herpes simplex virus-2 (HSV-2) transmission by 51%. We demonstrate that the concentration achieved intravaginally with a 1% tenofovir topical gel has direct antiherpetic activity. Tenofovir inhibits the replication of HSV clinical isolates in human embryonic fibroblasts, keratinocytes, and organotypic epithelial 3D rafts, decreases HSV replication in human lymphoid and cervicovaginal tissues ex vivo, and delays HSV-induced lesions and death in topically treated HSV-infected mice. The active tenofovir metabolite inhibits HSV DNA-polymerase and HIV reverse-transcriptase. To exert dual antiviral effects, tenofovir requires topical administration to achieve a drug concentration higher than systemic levels achieved by oral treatment. These findings indicate that a single topical treatment, like tenofovir, can inhibit the transmission of HIV and its copathogens.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Abdool Karim SS. Results of effectiveness trials of PRO 2000 gel: lessons for future microbicide trials. Future Microbiol. 2010;5:527–529. - PubMed
-
- Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 3rd ed. Garland Publishing; New York: 1994.
-
- Andrei G, Snoeck R, De Clercq E, Esnouf R, Fiten P, Opdenakker G. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 2000;81:639–648. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions

