Identification of an unique CXCR4 epitope whose ligation inhibits infection by both CXCR4 and CCR5 tropic human immunodeficiency type-I viruses
- PMID: 22018245
- PMCID: PMC3239297
- DOI: 10.1186/1742-4690-8-84
Identification of an unique CXCR4 epitope whose ligation inhibits infection by both CXCR4 and CCR5 tropic human immunodeficiency type-I viruses
Abstract
Background: Small chemical compounds which target chemokine receptors have been developed against human immunodeficiency virus type 1 (HIV-1) and are under investigation for use as anti-HIV-1 microbicides. In addition, monoclonal antibodies (mAbs) against chemokine receptors have also been shown to have anti-HIV-1 activities. The objective of the present study was to screen a panel of three anti-CXCR4 specific monoclonal antibodies (mAbs) for their ability to block the HIV-1 infection using in vitro activated primary peripheral blood mononuclear cells (PBMCs).
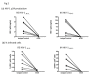
Results: PBMCs from normal donors were pre-activated with anti-CD3 and anti-CD28 mAbs for 1 day, and aliquots were infected with a low dose of CCR5-tropic (R5), CXCR4 tropic (X4) or dual tropic (X4R5) HIV-1 isolates and cultured in the presence of a panel of anti-CXCR4 mAbs. The panel included clones A145 mAb against the N-terminus, A120 mAb against a conformational epitope consisting of extracellular loops (ECL)1 and ECL2, and A80 mAb against ECL3 of CXCR4. Among these mAbs, the A120 mAb showed the most potent inhibition of infection, by not only X4 but surprisingly also R5 and X4R5 HIV-1. The inhibition of R5 HIV-1 was postulated to result from the novel ability of the A120 mAb to induce the levels of the CCR5-binding β-chemokines MIP-1α, MIP-1β and/or RANTES, and the down modulation of CCR5 expression on activated CD4+ T cells. Neutralizing anti-MIP-1α mAb significantly reversed the inhibitory effect of the A120 mAb on R5 HIV-1 infection.
Conclusions: The data described herein have identified a unique epitope of CXCR4 whose ligation not only directly inhibits X4 HIV-1, but also indirectly inhibits R5 HIV-1 infection by inducing higher levels of natural CCR5 ligands.
Figures




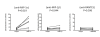


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

