An organ culture system to model early degenerative changes of the intervertebral disc
- PMID: 22018279
- PMCID: PMC3308106
- DOI: 10.1186/ar3494
An organ culture system to model early degenerative changes of the intervertebral disc
Abstract
Introduction: Back pain, a significant source of morbidity in our society, is related to the degenerative changes of the intervertebral disc. At present, the treatment of disc disease consists of therapies that are aimed at symptomatic relief. This shortcoming stems in large part from our lack of understanding of the biochemical and molecular events that drive the disease process. The goal of this study is to develop a model of early disc degeneration using an organ culture. This approach is based on our previous studies that indicate that organ culture closely models molecular events that occur in vivo in an ex vivo setting.
Methods: To mimic a degenerative insult, discs were cultured under low oxygen tension in the presence of TNF-α, IL-1β and serum limiting conditions.
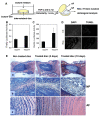
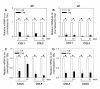
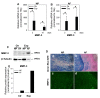
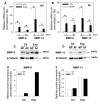
Results: Treatment resulted in compromised cell survival and changes in cellular morphology reminiscent of degeneration. There was strong suppression in the expression of matrix proteins including collagen types 1, 2, 6 and 9, proteoglycans, aggrecan and fibromodulin. Moreover, a strong induction in expression of catabolic matrix metalloproteinases (MMP) 3, 9 and 13 with a concomitant increase in aggrecan degradation was seen. An inductive effect on NGF expression was also noticed. Although similar, nucleus pulposus and annulus fibrosus tissues showed some differences in their response to the treatment.
Conclusions: Results of this study show that perturbations in microenvironmental factors result in anatomical and gene expression change within the intervertebral disc that may ultimately compromise cell function and induce pathological deficits. This system would be a valuable screening tool to investigate interventional strategies aimed at restoring disc cell function.
Figures

References
-
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

