Isolation and characterization of human spermatogonial stem cells
- PMID: 22018465
- PMCID: PMC3235066
- DOI: 10.1186/1477-7827-9-141
Isolation and characterization of human spermatogonial stem cells
Abstract
Background: To isolate and characterization of human spermatogonial stem cells from stem spermatogonium.
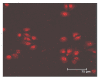
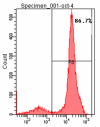
Methods: The disassociation of spermatogonial stem cells (SSCs) were performed using enzymatic digestion of type I collagenase and trypsin. The SSCs were isolated by using Percoll density gradient centrifugation, followed by differential surface-attachment method. Octamer-4(OCT4)-positive SSC cells were further identified using immunofluorescence staining and flow cytometry technques. The purity of the human SSCs was also determined, and a co-culture system for SSCs and Sertoli cells was established.
Results: The cell viability was 91.07% for the suspension of human spermatogonial stem cells dissociated using a two-step enzymatic digestion process. The cells isolated from Percoll density gradient coupled with differential surface-attachement purification were OCT4 positive, indicating the cells were human spermatogonial stem cells. The purity of isolated human spermatogonial stem cells was 86.7% as assessed by flow cytometry. The isolated SSCs were shown to form stable human spermatogonial stem cell colonies on the feeder layer of the Sertoli cells.
Conclusions: The two-step enzyme digestion (by type I collagenase and trypsin) process is an economical, simple and reproducible technique for isolating human spermatogonial stem cells. With little contamination and less cell damage, this method facilitates isolated human spermatogonial stem cells to form a stable cell colony on the supporting cell layer.
Figures




References
-
- Mirzapour T, Movahedin M, Tengku Ibrahim TA, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2011. - PubMed
-
- Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, Yuen C, Greilach S, Zhao HH, Chow M, Chow YC, Rao J, Barritt J, Bar-Chama N, Copperman A. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26:1296–1306. doi: 10.1093/humrep/der026. - DOI - PubMed
-
- Gang B, Yan-feng L, Qian-sheng L, Feng-shuo J, Yong Z. Isolation and purification of human spermatogenous cells. Acta Academiae Medicinae Militaris Tertiae. 2005;27:1142–1144.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

