Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up
- PMID: 22018631
- PMCID: PMC3235950
- DOI: 10.1016/S1470-2045(11)70270-4
Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up
Abstract
Background: Postmenopausal women with hormone receptor-positive early breast cancer have persistent, long-term risk of breast-cancer recurrence and death. Therefore, trials assessing endocrine therapies for this patient population need extended follow-up. We present an update of efficacy outcomes in the Breast International Group (BIG) 1-98 study at 8·1 years median follow-up.
Methods: BIG 1-98 is a randomised, phase 3, double-blind trial of postmenopausal women with hormone receptor-positive early breast cancer that compares 5 years of tamoxifen or letrozole monotherapy, or sequential treatment with 2 years of one of these drugs followed by 3 years of the other. Randomisation was done with permuted blocks, and stratified according to the two-arm or four-arm randomisation option, participating institution, and chemotherapy use. Patients, investigators, data managers, and medical reviewers were masked. The primary efficacy endpoint was disease-free survival (events were invasive breast cancer relapse, second primaries [contralateral breast and non-breast], or death without previous cancer event). Secondary endpoints were overall survival, distant recurrence-free interval (DRFI), and breast cancer-free interval (BCFI). The monotherapy comparison included patients randomly assigned to tamoxifen or letrozole for 5 years. In 2005, after a significant disease-free survival benefit was reported for letrozole as compared with tamoxifen, a protocol amendment facilitated the crossover to letrozole of patients who were still receiving tamoxifen alone; Cox models and Kaplan-Meier estimates with inverse probability of censoring weighting (IPCW) are used to account for selective crossover to letrozole of patients (n=619) in the tamoxifen arm. Comparison of sequential treatments to letrozole monotherapy included patients enrolled and randomly assigned to letrozole for 5 years, letrozole for 2 years followed by tamoxifen for 3 years, or tamoxifen for 2 years followed by letrozole for 3 years. Treatment has ended for all patients and detailed safety results for adverse events that occurred during the 5 years of treatment have been reported elsewhere. Follow-up is continuing for those enrolled in the four-arm option. BIG 1-98 is registered at clinicaltrials.govNCT00004205.
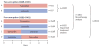
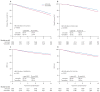
Findings: 8010 patients were included in the trial, with a median follow-up of 8·1 years (range 0-12·4). 2459 were randomly assigned to monotherapy with tamoxifen for 5 years and 2463 to monotherapy with letrozole for 5 years. In the four-arm option of the trial, 1546 were randomly assigned to letrozole for 5 years, 1548 to tamoxifen for 5 years, 1540 to letrozole for 2 years followed by tamoxifen for 3 years, and 1548 to tamoxifen for 2 years followed by letrozole for 3 years. At a median follow-up of 8·7 years from randomisation (range 0-12·4), letrozole monotherapy was significantly better than tamoxifen, whether by IPCW or intention-to-treat analysis (IPCW disease-free survival HR 0·82 [95% CI 0·74-0·92], overall survival HR 0·79 [0·69-0·90], DRFI HR 0·79 [0·68-0·92], BCFI HR 0·80 [0·70-0·92]; intention-to-treat disease-free survival HR 0·86 [0·78-0·96], overall survival HR 0·87 [0·77-0·999], DRFI HR 0·86 [0·74-0·998], BCFI HR 0·86 [0·76-0·98]). At a median follow-up of 8·0 years from randomisation (range 0-11·2) for the comparison of the sequential groups with letrozole monotherapy, there were no statistically significant differences in any of the four endpoints for either sequence. 8-year intention-to-treat estimates (each with SE ≤1·1%) for letrozole monotherapy, letrozole followed by tamoxifen, and tamoxifen followed by letrozole were 78·6%, 77·8%, 77·3% for disease-free survival; 87·5%, 87·7%, 85·9% for overall survival; 89·9%, 88·7%, 88·1% for DRFI; and 86·1%, 85·3%, 84·3% for BCFI.
Interpretation: For postmenopausal women with endocrine-responsive early breast cancer, a reduction in breast cancer recurrence and mortality is obtained by letrozole monotherapy when compared with tamoxifen montherapy. Sequential treatments involving tamoxifen and letrozole do not improve outcome compared with letrozole monotherapy, but might be useful strategies when considering an individual patient's risk of recurrence and treatment tolerability.
Funding: Novartis, United States National Cancer Institute, International Breast Cancer Study Group.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No medical writer or editor was involved in this paper.
Figures


References
-
- BIG 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. - PubMed
-
- Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25:486–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

