Innovative therapy for Classic Galactosemia - tale of two HTS
- PMID: 22018723
- PMCID: PMC3253915
- DOI: 10.1016/j.ymgme.2011.09.028
Innovative therapy for Classic Galactosemia - tale of two HTS
Abstract
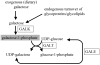
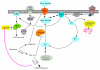
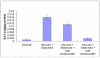
Classic Galactosemia is an autosomal recessive disorder caused by the deficiency of galactose-1-phosphate uridylyltransferase (GALT), one of the key enzymes in the Leloir pathway of galactose metabolism. While the neonatal morbidity and mortality of the disease are now mostly prevented by newborn screening and galactose restriction, long-term outcome for older children and adults with this disorder remains unsatisfactory. The pathophysiology of Classic Galactosemia is complex, but there is convincing evidence that galactose-1-phosphate (gal-1P) accumulation is a major, if not the sole pathogenic factor. Galactokinase (GALK) inhibition will eliminate the accumulation of gal-1P from both dietary sources and endogenous production, and efforts toward identification of therapeutic small molecule GALK inhibitors are reviewed in detail. Experimental and computational high-throughput screenings of compound libraries to identify GALK inhibitors have been conducted, and subsequent studies aimed to characterize, prioritize, as well as to optimize the identified positives have been implemented to improve the potency of promising compounds. Although none of the identified GALK inhibitors inhibits glucokinase and hexokinase, some of them cross-inhibit other related enzymes in the GHMP small molecule kinase superfamily. While this finding may render the on-going hit-to-lead process more challenging, there is growing evidence that such cross-inhibition could also lead to advances in antimicrobial and anti-cancer therapies.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



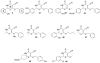
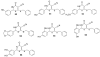
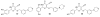
References
-
- Leloir LF. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem. 1951;33(2):186–90. - PubMed
-
- Acosta PB, Gross KC. Hidden sources of galactose in the environment. Eur J Pediatr. 1995;154(7 Suppl 2):S87–92. - PubMed
-
- Berry GT, et al. The effect of dietary fruits and vegetables on urinary galactitol excretion in galactose-1-phosphate uridyltransferase deficiency. J Inherit Metab Dis. 1993;16(1):91–100. - PubMed
-
- Berry GT, et al. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet. 1995;346(8982):1073–4. - PubMed
-
- Berry GT, et al. The rate of de novo galactose synthesis in patients with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab. 2004;81(1):22–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

