Squirrel monkeys (Saimiri sciureus) infected with the agent of bovine spongiform encephalopathy develop tau pathology
- PMID: 22018806
- PMCID: PMC3288625
- DOI: 10.1016/j.jcpa.2011.09.004
Squirrel monkeys (Saimiri sciureus) infected with the agent of bovine spongiform encephalopathy develop tau pathology
Abstract
Squirrel monkeys (Saimiri sciureus) were infected experimentally with the agent of classical bovine spongiform encephalopathy (BSE). Two to four years later, six of the monkeys developed alterations in interactive behaviour and cognition and other neurological signs typical of transmissible spongiform encephalopathy (TSE). At necropsy examination, the brains from all of the monkeys showed pathological changes similar to those described in variant Creutzfeldt-Jakob disease (vCJD) of man, except that the squirrel monkey brains contained no PrP-amyloid plaques typical of that disease. Constant neuropathological features included spongiform degeneration, gliosis, deposition of abnormal prion protein (PrP(TSE)) and many deposits of abnormally phosphorylated tau protein (p-Tau) in several areas of the cerebrum and cerebellum. Western blots showed large amounts of proteinase K-resistant prion protein in the central nervous system. The striking absence of PrP plaques (prominent in brains of cynomolgus macaques [Macaca fascicularis] with experimentally-induced BSE and vCJD and in human patients with vCJD) reinforces the conclusion that the host plays a major role in determining the neuropathology of TSEs. Results of this study suggest that p-Tau, found in the brains of all BSE-infected monkeys, might play a role in the pathogenesis of TSEs. Whether p-Tau contributes to development of disease or appears as a secondary change late in the course of illness remains to be determined.
Published by Elsevier Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
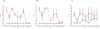


References
-
- Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. - PubMed
-
- Aho L, Pikkarainen M, Hiltunen M, Leinonen V, Alafuzoff I. Immunohistochemical visualization of amyloid-β protein precursor and amyloid β in extra- and intracellular compartments in human brain. Journal of Alzheimer’s Disease. 2010;20:1015–1028. - PubMed
-
- Alzualde A, Indakoetxea B, Ferrer I, Moreno F, Barandiaran M, et al. A novel PRNP Y218N mutation in Gertsmann-Sträussler-Scheinker disease with neurofibrillary degeneration. Journal of Neuropathology and Experimental Neurology. 2010;69:789–800. - PubMed
-
- Asher DM, Gibbs CJ, Jr, Sulima MP, Bacote A, Gajdusek DC. Transmission of human spongiform encephalopathies to experimental animals: Comparison of the chimpanzee and squirrel monkey. In: Brown F, editor. Transmissible Spongiform Encephalopathies - Impact on Animal and Human Health. Developments in Biological Standardization. Vol. 80. Basel: Karger; 1993. pp. 9–13. - PubMed
-
- Asuni AA, Perry VH, O’Connor V. Change in tau phosphorylation associated with neurodegeneration in the ME7 model of prion disease. Biochemical Society Transactions. 2010;38:545–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

