In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives
- PMID: 22019045
- PMCID: PMC3383042
- DOI: 10.1016/j.bmc.2011.09.051
In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives
Abstract
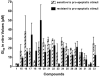
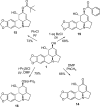
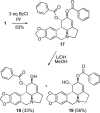
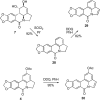
As a continuation of our studies aimed at the development of a new cytostatic agent derived from an Amaryllidaceae alkaloid lycorine, we synthesized 32 analogues of this natural product. This set of synthetic analogues included compounds incorporating selective derivatization of the C1 versus C2 hydroxyl groups, aromatized ring C, lactamized N6 nitrogen, dihydroxylated C3-C3a olefin functionality, transposed olefin from C3-C3a to C2-C3 or C3a-C4, and C1 long-chain fatty esters. All synthesized compounds were evaluated for antiproliferative activities in vitro in a panel of tumor cell lines including those exhibiting resistance to proapoptotic stimuli and representing solid cancers associated with dismal prognoses, such as melanoma, glioblastoma, and non-small-cell lung cancer. Most active analogues were not discriminatory between cancer cells displaying resistance or sensitivity to apoptosis, indicating that these compounds are thus able to overcome the intrinsic resistance of cancer cells to pro-apoptotic stimuli. 1,2-Di-O-allyllycorine was identified as a lycorine analogue, which is 100 times more potent against a U373 human glioblastoma model than the parent natural product. Furthermore, a number of synthetic analogues were identified as promising for the forthcoming in vivo studies.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
C1,C2-ether derivatives of the Amaryllidaceae alkaloid lycorine: retention of activity of highly lipophilic analogues against cancer cells.Bioorg Med Chem Lett. 2014 Feb 1;24(3):923-7. doi: 10.1016/j.bmcl.2013.12.073. Epub 2013 Dec 24. Bioorg Med Chem Lett. 2014. PMID: 24393582
-
Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: an investigation of structure-activity relationship and mechanistic insight.J Med Chem. 2009 Oct 22;52(20):6244-56. doi: 10.1021/jm901031h. J Med Chem. 2009. PMID: 19788245 Free PMC article.
-
Structure-activity studies on the lycorine pharmacophore: A potent inducer of apoptosis in human leukemia cells.Phytochemistry. 2009 May;70(7):913-9. doi: 10.1016/j.phytochem.2009.04.012. Epub 2009 May 20. Phytochemistry. 2009. PMID: 19464034 Free PMC article.
-
Cytotoxic lycorine alkaloids of the plant family Amaryllidaceae.Bioorg Chem. 2025 Aug;163:108619. doi: 10.1016/j.bioorg.2025.108619. Epub 2025 May 23. Bioorg Chem. 2025. PMID: 40516169 Review.
-
Lycorine and its derivatives for anticancer drug design.Mini Rev Med Chem. 2010 Jan;10(1):41-50. doi: 10.2174/138955710791112604. Mini Rev Med Chem. 2010. PMID: 20105122 Review.
Cited by
-
Lycorine inhibits glioblastoma multiforme growth through EGFR suppression.J Exp Clin Cancer Res. 2018 Jul 17;37(1):157. doi: 10.1186/s13046-018-0785-4. J Exp Clin Cancer Res. 2018. PMID: 30016965 Free PMC article.
-
Isatin derivatives with activity against apoptosis-resistant cancer cells.Bioorg Med Chem Lett. 2016 Mar 15;26(6):1558-1560. doi: 10.1016/j.bmcl.2016.02.015. Epub 2016 Feb 8. Bioorg Med Chem Lett. 2016. PMID: 26883150 Free PMC article.
-
Fungal metabolite ophiobolin A as a promising anti-glioma agent: In vivo evaluation, structure-activity relationship and unique pyrrolylation of primary amines.Bioorg Med Chem Lett. 2015 Oct 15;25(20):4544-8. doi: 10.1016/j.bmcl.2015.08.066. Epub 2015 Aug 24. Bioorg Med Chem Lett. 2015. PMID: 26341136 Free PMC article.
-
Activity of 2-aryl-2-(3-indolyl)acetohydroxamates against drug-resistant cancer cells.J Med Chem. 2015 Mar 12;58(5):2206-20. doi: 10.1021/jm501518y. Epub 2015 Feb 20. J Med Chem. 2015. PMID: 25671501 Free PMC article.
-
Preparation of Structurally Diverse Compounds from the Natural Product Lycorine.Org Lett. 2018 Sep 21;20(18):5894-5898. doi: 10.1021/acs.orglett.8b02562. Epub 2018 Sep 11. Org Lett. 2018. PMID: 30204451 Free PMC article.
References
-
- Lefranc F, Brotchi J, Kiss R. J. Clin. Oncol. 2005;23:2411–2422. - PubMed
-
- La Porta CA. Curr. Med. Chem. 2007;14:387–391. - PubMed
-
- D'Amico TA, Harpole DH., Jr. Chest Surg. Clin. N. Am. 2000;10:451–469. - PubMed
-
- Fenell DA. Clin. Cancer Res. 2005;11:2097–2105. - PubMed
-
-
For some recent reports, see: McNulty J, Nair JJ, Little JRL, Brennan JD, Bastida J. Bioorg. Med. Chem. Lett. 2010;20:5290–5294.. Cedron JC, Gutierrez D, Flores N, Ravelo AG, Estevez-Braun A. Bioorg. Med. Chem. 2010;18:4694–4701..
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

