The maternal immune response to fetal platelet GPIbα causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies
- PMID: 22019589
- PMCID: PMC3204841
- DOI: 10.1172/JCI57850
The maternal immune response to fetal platelet GPIbα causes frequent miscarriage in mice that can be prevented by intravenous IgG and anti-FcRn therapies
Abstract
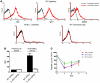
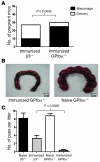
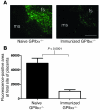
Fetal and neonatal immune thrombocytopenia (FNIT) is a severe bleeding disorder caused by maternal antibody-mediated destruction of fetal/neonatal platelets. It is the most common cause of severe thrombocytopenia in neonates, but the frequency of FNIT-related miscarriage is unknown, and the mechanism(s) underlying fetal mortality have not been explored. Furthermore, although platelet αIIbβ3 integrin and GPIbα are the major antibody targets in immune thrombocytopenia, the reported incidence of anti-GPIbα-mediated FNIT is rare. Here, we developed mouse models of FNIT mediated by antibodies specific for GPIbα and β3 integrin and compared their pathogenesis. We found, unexpectedly, that miscarriage occurred in the majority of pregnancies in our model of anti-GPIbα-mediated FNIT, which was far more frequent than in anti-β3-mediated FNIT. Dams with anti-GPIbα antibodies exhibited extensive fibrin deposition and apoptosis/necrosis in their placentas, which severely impaired placental function. Furthermore, anti-GPIbα (but not anti-β3) antiserum activated platelets and enhanced fibrin formation in vitro and thrombus formation in vivo. Importantly, treatment with either intravenous IgG or a monoclonal antibody specific for the neonatal Fc receptor efficiently prevented anti-GPIbα-mediated FNIT. Thus, the maternal immune response to fetal GPIbα causes what we believe to be a previously unidentified, nonclassical FNIT (i.e., spontaneous miscarriage but not neonatal bleeding) in mice. These results suggest that a similar pathology may have masked the severity and frequency of human anti-GPIbα-mediated FNIT, but also point to possible therapeutic interventions.
Figures





Comment in
-
Are maternal antiplatelet antibodies a prothrombotic condition leading to miscarriage?J Clin Invest. 2011 Nov;121(11):4241-3. doi: 10.1172/JCI60749. Epub 2011 Oct 24. J Clin Invest. 2011. PMID: 22019585 Free PMC article.
References
-
- Dreyfus M, Kaplan C, Verdy E, Schlegel N, Durand-Zaleski I, Tchernia G. Frequency of immune thrombocytopenia in newborns: a prospective study. Immune Thrombocytopenia Working Group. Blood. 1997;89(12):4402–4406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

