Glial-derived neurotrophic factor gene transfer for Parkinson's disease: anterograde distribution of AAV2 vectors in the primate brain
- PMID: 22019719
- PMCID: PMC3289735
- DOI: 10.1016/j.nbd.2011.10.004
Glial-derived neurotrophic factor gene transfer for Parkinson's disease: anterograde distribution of AAV2 vectors in the primate brain
Abstract
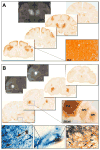
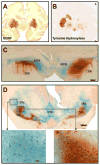
Delivery of neurotrophic factors to treat neurodegenerative diseases has not been efficacious in clinical trials despite their known potency for promoting neuronal growth and survival. Direct gene delivery to the brain offers an approach for establishing sustained expression of neurotrophic factors but is dependent on accurate surgical procedures to target specific anatomical regions of the brain. Serotype-2 adeno-associated viral (AAV2) vectors have been investigated in multiple clinical studies for neurological diseases without adverse effects; however the absence of significant clinical efficacy after neurotrophic factor gene transfer has been largely attributed to insufficient coverage of the target region. Our pre-clinical development of AAV2-glial-derived neurotrophic factor (GDNF) for Parkinson's disease involved real-time image guided delivery and optimization of delivery techniques to maximize gene transfer in the putamen. We have demonstrated that AAV2 vectors are anterogradely transported in the primate brain with GDNF expression observed in the substantia nigra after putaminal delivery in both intact and nigrostriatal lesioned primates. Direct midbrain delivery of AAV2-GDNF resulted in extensive anterograde transport to multiple brain regions and significant weight loss.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. - PubMed
-
- Cattaneo A, et al. Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J Alzheimers Dis. 2008;15:255–83. - PubMed
-
- Choi-Lundberg DL, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–41. - PubMed
-
- Choi-Lundberg DL, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

