Cadmium exposure activates the ERK signaling pathway leading to altered osteoblast gene expression and apoptotic death in Saos-2 cells
- PMID: 22019892
- PMCID: PMC3267888
- DOI: 10.1016/j.fct.2011.10.031
Cadmium exposure activates the ERK signaling pathway leading to altered osteoblast gene expression and apoptotic death in Saos-2 cells
Abstract
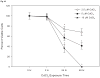
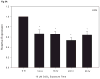
Recent reports of cadmium in electronic waste and jewelry have increased public awareness regarding this toxic metal. Human exposure to cadmium is associated with the development of osteoporosis. We previously reported cadmium induces apoptosis in human tumor-derived Saos-2 osteoblasts. In this study, we examine the extracellular signal-regulated protein kinase (ERK) and protein kinase C (PKC) pathways in cadmium-induced apoptosis and altered osteoblast gene expression. Saos-2 osteoblasts were cultured in the presence or absence of 10μM CdCl(2) for 2-72h. We detected significant ERK activation in response to CdCl(2) and pretreatment with the ERK inhibitor PD98059 attenuated cadmium-induced apoptosis. However, PKCα activation was not observed after exposure to CdCl(2) and pretreatment with the PKC inhibitor, Calphostin C, was unable to rescue cells from cadmium-induced apoptosis. Gene expression studies were conducted using qPCR. Cells exposed to CdCl(2) exhibited a significant decrease in the bone-forming genes osteopontin (OPN) and alkaline phosphatase (ALP) mRNA. In contrast, SOST, whose protein product inhibits bone formation, significantly increased in response to CdCl(2). Pretreatment with PD98059 had a recovery effect on cadmium-induced changes in gene expression. This research demonstrates cadmium can directly inhibit osteoblasts via ERK signaling pathway and identifies SOST as a target for cadmium-induced osteotoxicity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



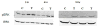
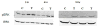








References
-
- Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate Inhibits Mineralization of Osteoblast Cultures by Binding to Mineral, Up-regulating Osteopontin, and Inhibiting Alkaline Phosphatase Activity. Journal of Biological Chemistry. 2007;282(21):15872–15883. - PubMed
-
- Bai X, Lu D, Bai J, Zheng H, Ke Z, Li X, Luo S. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochemical and Biophysical Research Communications. 2004;314:197–207. - PubMed
-
- Bodo M, Balloni S, Lumare E, Bacci M, Calvitti M, Dell’Omo M, Murgia N, Marinucci L. Effects of sub-toxic Cadmium concentrations on bone gene expression program: Results of an in vitro study. Toxicology in Vitro. 2010;6:1670–1680. - PubMed
-
- Bradford M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

