Induction of DNA damage in human urothelial cells by the brominated flame retardant 2,2-bis(bromomethyl)-1,3-propanediol: role of oxidative stress
- PMID: 22019925
- PMCID: PMC3248618
- DOI: 10.1016/j.tox.2011.10.006
Induction of DNA damage in human urothelial cells by the brominated flame retardant 2,2-bis(bromomethyl)-1,3-propanediol: role of oxidative stress
Abstract
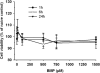
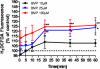
2,2-bis(bromomethyl)-1,3-propanediol (BMP) is an extensively used brominated flame retardant found in urethane foams and polyester resins. In a 2-year dietary study conducted by the National Toxicology Program, BMP caused neoplastic lesions at multiple sites including the urinary bladder in both rats and mice. The mechanism of its carcinogenic effect is unknown. In the present study, using SV-40 immortalized human urothelial cells (UROtsa), endpoints associated with BMP induced DNA damage and oxidative stress were investigated. The effects of time (1-24h) and concentration (5-100 μM) on BMP induced DNA strand breaks were assessed via the alkaline comet assay. The results revealed evidence of DNA strand breaks at 1 and 3h following incubation of cells with non-cytotoxic concentrations of BMP. Strand breaks were not present after 6h of incubation. Evidences for BMP associated oxidative stress include: an elevation of intracellular ROS formation as well as induction of Nrf2 and HSP70 protein levels. In addition, DNA strand breaks were attenuated when cells were pre-treated with N-acetyl-l-cysteine (NAC) and oxidative base modifications were revealed when a lesion specific endonuclease, human 8-hydroxyguanine DNA glycosylase 1 (hOGG1) was introduced into the comet assay. In conclusion, these results demonstrate that BMP induces DNA strand breaks and oxidative base damage in UROtsa cells. Oxidative stress is a significant, determinant factor in mediating these DNA lesions. These early genotoxic events may, in part, contribute to BMP-induced carcinogenesis observed in rodents.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures




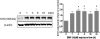
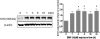

Similar articles
-
Comparison of 2,2-bis(bromomethyl)-1,3-propanediol induced genotoxicity in UROtsa cells and primary rat hepatocytes: relevance of metabolism and oxidative stress.Toxicol Lett. 2013 Oct 9;222(3):273-9. doi: 10.1016/j.toxlet.2013.07.026. Epub 2013 Aug 13. Toxicol Lett. 2013. PMID: 23954263 Free PMC article.
-
Functional genomic assessment of 2, 2-bis (bromomethyl)-1, 3-propanediol induced cytotoxicity in a single-gene knockout library of E. coli.Chemosphere. 2017 Oct;185:582-588. doi: 10.1016/j.chemosphere.2017.07.031. Epub 2017 Jul 9. Chemosphere. 2017. PMID: 28719877
-
Effects of seven chemicals on DNA damage in the rat urinary bladder: a comet assay study.Mutat Res Genet Toxicol Environ Mutagen. 2014 Jul 15;769:1-6. doi: 10.1016/j.mrgentox.2014.04.015. Epub 2014 May 10. Mutat Res Genet Toxicol Environ Mutagen. 2014. PMID: 25344106
-
Genotoxicity of environmental agents assessed by the alkaline comet assay.Basic Clin Pharmacol Toxicol. 2005;96 Suppl 1:1-42. Basic Clin Pharmacol Toxicol. 2005. PMID: 15859009 Review.
-
Assessment of evidence for nanosized titanium dioxide-generated DNA strand breaks and oxidatively damaged DNA in cells and animal models.Nanotoxicology. 2017 Nov-Dec;11(9-10):1237-1256. doi: 10.1080/17435390.2017.1406549. Epub 2017 Nov 27. Nanotoxicology. 2017. PMID: 29172839 Review.
Cited by
-
Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation.PLoS One. 2014 May 7;9(5):e94298. doi: 10.1371/journal.pone.0094298. eCollection 2014. PLoS One. 2014. PMID: 24804719 Free PMC article.
-
Heat shock protein 70 (Hsp70) inhibits oxidative phosphorylation and compensates ATP balance through enhanced glycolytic activity.J Appl Physiol (1985). 2012 Dec 1;113(11):1669-76. doi: 10.1152/japplphysiol.00658.2012. Epub 2012 Oct 4. J Appl Physiol (1985). 2012. PMID: 23042904 Free PMC article.
-
New exposure biomarkers as tools for breast cancer epidemiology, biomonitoring, and prevention: a systematic approach based on animal evidence.Environ Health Perspect. 2014 Sep;122(9):881-95. doi: 10.1289/ehp.1307455. Epub 2014 May 12. Environ Health Perspect. 2014. PMID: 24818537 Free PMC article. Review.
-
Exposure of Female Rats to an Environmentally Relevant Mixture of Brominated Flame Retardants Targets the Ovary, Affecting Folliculogenesis and Steroidogenesis.Biol Reprod. 2016 Jan;94(1):9. doi: 10.1095/biolreprod.115.134452. Epub 2015 Nov 25. Biol Reprod. 2016. PMID: 26607716 Free PMC article.
-
Comparison of 2,2-bis(bromomethyl)-1,3-propanediol induced genotoxicity in UROtsa cells and primary rat hepatocytes: relevance of metabolism and oxidative stress.Toxicol Lett. 2013 Oct 9;222(3):273-9. doi: 10.1016/j.toxlet.2013.07.026. Epub 2013 Aug 13. Toxicol Lett. 2013. PMID: 23954263 Free PMC article.
References
-
- Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010;49:9–21. - PubMed
-
- Collins AR, Ma AG, Duthie SJ. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995;336:69–77. - PubMed
-
- Crespan E, Amoroso A, Maga G. DNA polymerases and mutagenesis in human cancers. Subcell. Biochem. 2010;50:165–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

