Neutrophil-cytokine interactions in a rat model of sulindac-induced idiosyncratic liver injury
- PMID: 22019926
- PMCID: PMC3226905
- DOI: 10.1016/j.tox.2011.10.005
Neutrophil-cytokine interactions in a rat model of sulindac-induced idiosyncratic liver injury
Abstract
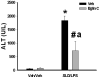
Previous studies indicated that lipopolysaccharide (LPS) interacts with the nonsteroidal anti-inflammatory drug sulindac (SLD) to produce liver injury in rats. In the present study, the mechanism of SLD/LPS-induced liver injury was further investigated. Accumulation of polymorphonuclear neutrophils (PMNs) in the liver was greater in SLD/LPS-cotreated rats compared to those treated with SLD or LPS alone. In addition, PMN activation occurred specifically in livers of rats cotreated with SLD/LPS. The hypothesis that PMNs and proteases released from them play critical roles in the hepatotoxicity was tested. SLD/LPS-induced liver injury was attenuated by prior depletion of PMNs or by treatment with the PMN protease inhibitor, eglin C. Previous studies suggested that tumor necrosis factor-α (TNF) and the hemostatic system play critical roles in the pathogenesis of liver injury induced by SLD/LPS. TNF and plasminogen activator inhibitor-1 (PAI-1) can contribute to hepatotoxicity by affecting PMN activation and fibrin deposition. Therefore, the role of TNF and PAI-1 in PMN activation and fibrin deposition in the SLD/LPS-induced liver injury model was tested. Neutralization of TNF or inhibition of PAI-1 attenuated PMN activation. TNF had no effect on PAI-1 production or fibrin deposition. In contrast, PAI-1 contributed to fibrin deposition in livers of rats treated with SLD/LPS. In summary, PMNs, TNF and PAI-1 contribute to the liver injury induced by SLD/LPS cotreatment. TNF and PAI-1 independently contributed to PMN activation, which is critical to the pathogenesis of liver injury. Moreover, PAI-1 contributed to liver injury by promoting fibrin deposition.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
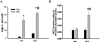
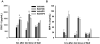

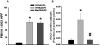




Similar articles
-
Roles of the hemostatic system and neutrophils in liver injury from co-exposure to amiodarone and lipopolysaccharide.Toxicol Sci. 2013 Nov;136(1):51-62. doi: 10.1093/toxsci/kft170. Epub 2013 Aug 2. Toxicol Sci. 2013. PMID: 23912913 Free PMC article.
-
Neutrophil interaction with the hemostatic system contributes to liver injury in rats cotreated with lipopolysaccharide and ranitidine.J Pharmacol Exp Ther. 2007 Aug;322(2):852-61. doi: 10.1124/jpet.107.122069. Epub 2007 May 15. J Pharmacol Exp Ther. 2007. PMID: 17505017 Free PMC article.
-
Oxidative stress is important in the pathogenesis of liver injury induced by sulindac and lipopolysaccharide cotreatment.Toxicology. 2010 Jun 4;272(1-3):32-8. doi: 10.1016/j.tox.2010.03.015. Epub 2010 Apr 3. Toxicology. 2010. PMID: 20371263 Free PMC article.
-
Hepatotoxic interaction of sulindac with lipopolysaccharide: role of the hemostatic system.Toxicol Sci. 2009 Mar;108(1):184-93. doi: 10.1093/toxsci/kfn259. Epub 2008 Dec 12. Toxicol Sci. 2009. PMID: 19074762 Free PMC article.
-
Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism.Exp Biol Med (Maywood). 2012 Jan;237(1):1-9. doi: 10.1258/ebm.2011.011255. Exp Biol Med (Maywood). 2012. PMID: 22238286 Free PMC article. Review.
Cited by
-
Involvement of TGF-β1/Smad3 Signaling in Carbon Tetrachloride-Induced Acute Liver Injury in Mice.PLoS One. 2016 May 25;11(5):e0156090. doi: 10.1371/journal.pone.0156090. eCollection 2016. PLoS One. 2016. PMID: 27224286 Free PMC article.
-
Roles of the hemostatic system and neutrophils in liver injury from co-exposure to amiodarone and lipopolysaccharide.Toxicol Sci. 2013 Nov;136(1):51-62. doi: 10.1093/toxsci/kft170. Epub 2013 Aug 2. Toxicol Sci. 2013. PMID: 23912913 Free PMC article.
-
Treprostinil alleviates hepatic mitochondrial injury during rat renal ischemia-reperfusion injury.Biomed Pharmacother. 2021 Nov;143:112172. doi: 10.1016/j.biopha.2021.112172. Epub 2021 Sep 21. Biomed Pharmacother. 2021. PMID: 34560548 Free PMC article.
-
Idiosyncratic Drug-Induced Liver Injury (IDILI): Potential Mechanisms and Predictive Assays.Biomed Res Int. 2017;2017:9176937. doi: 10.1155/2017/9176937. Epub 2017 Jan 4. Biomed Res Int. 2017. PMID: 28133614 Free PMC article. Review.
-
Halogenation Activity of Mammalian Heme Peroxidases.Antioxidants (Basel). 2022 Apr 30;11(5):890. doi: 10.3390/antiox11050890. Antioxidants (Basel). 2022. PMID: 35624754 Free PMC article. Review.
References
-
- Baranes D, Matzner J, Razin E. Thrombin-induced calcium-independent degranulation of human neutrophils. Inflammation. 1986;10:455–461. - PubMed
-
- Braun NJ, Bodmer JL, Virca GD, Metz-Virca G, Maschler R, Bieth JG, Schnebli HP. Kinetic studies on the interaction of eglin c with human leukocyte elastase and cathepsin G. Biol Chem Hoppe Seyler. 1987;368:299–308. - PubMed
-
- Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–1200. - PubMed
-
- Copple BL, Moulin F, Hanumegowda UM, Ganey PE, Roth RA. Thrombin and protease-activated receptor-1 agonists promote lipopolysaccharide-induced hepatocellular injury in perfused livers. J Pharmacol Exp Ther. 2003;305:417–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

