Electrical signaling in control of ocular cell behaviors
- PMID: 22020127
- PMCID: PMC3242826
- DOI: 10.1016/j.preteyeres.2011.10.001
Electrical signaling in control of ocular cell behaviors
Abstract
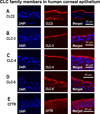
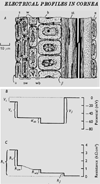
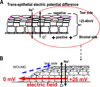
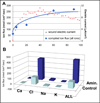
Epithelia of the cornea, lens and retina contain a vast array of ion channels and pumps. Together they produce a polarized flow of ions in and out of cells, as well as across the epithelia. These naturally occurring ion fluxes are essential to the hydration and metabolism of the ocular tissues, especially for the avascular cornea and lens. The directional transport of ions generates electric fields and currents in those tissues. Applied electric fields affect migration, division and proliferation of ocular cells which are important in homeostasis and healing of the ocular tissues. Abnormalities in any of those aspects may underlie many ocular diseases, for example chronic corneal ulcers, posterior capsule opacity after cataract surgery, and retinopathies. Electric field-inducing cellular responses, termed electrical signaling here, therefore may be an unexpected yet powerful mechanism in regulating ocular cell behavior. Both endogenous electric fields and applied electric fields could be exploited to regulate ocular cells. We aim to briefly describe the physiology of the naturally occurring electrical activities in the corneal, lens, and retinal epithelia, to provide experimental evidence of the effects of electric fields on ocular cell behaviors, and to suggest possible clinical implications.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures













References
-
- Akaike N, Kiyohara T. Metabolic component in the epithelial intracellular potential of rabbit cornea. Pflugers Arch. 1977;369:33–37. - PubMed
-
- Al-Nakkash L, Iserovich P, Coca-Prados M, Yang H, Reinach PS. Functional and molecular characterization of a volume-activated chloride channel in rabbit corneal epithelial cells. J Membr Biol. 2004;201:41–49. - PubMed
-
- Al-Nakkash L, Reinach PS. Activation of a CFTR-mediated chloride current in a rabbit corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2001;42:2364–2370. - PubMed
-
- Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, Tsai JC, Castaneda VE, Hoggatt JP, Kostick AM. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. - PubMed
-
- Baehr W, Wu SM, Bird AC, Palczewski K. The retinoid cycle and retina disease. Vision Res. 2003;43:2957–2958. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

