Novel cancer immunotherapy agents with survival benefit: recent successes and next steps
- PMID: 22020206
- PMCID: PMC3426440
- DOI: 10.1038/nrc3153
Novel cancer immunotherapy agents with survival benefit: recent successes and next steps
Abstract
The US Food and Drug Administration (FDA) recently approved two novel immunotherapy agents, sipuleucel-T and ipilimumab, which showed a survival benefit for patients with metastatic prostate cancer and melanoma, respectively. The mechanisms by which these agents provideclinical benefit are not completely understood. However, knowledge of these mechanisms will be crucial for probing human immune responses and tumour biology in order to understand what distinguishes responders from non-responders. The following next steps are necessary: first, the development of immune-monitoring strategies for the identification of relevant biomarkers; second, the establishment of guidelines for the assessment of clinical end points; and third, the evaluation of combination therapy strategies to improve clinical benefit.
Figures

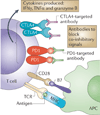

References
-
- Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1:1171–1174. - PubMed
-
- Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. - PubMed
-
- Shankaran V, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

