Development and distribution of neuronal cilia in mouse neocortex
- PMID: 22020803
- PMCID: PMC3325766
- DOI: 10.1002/cne.22793
Development and distribution of neuronal cilia in mouse neocortex
Abstract
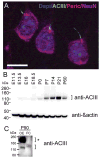
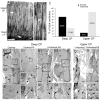
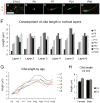
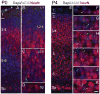
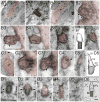
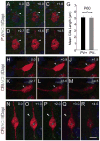
Neuronal primary cilia are not generally recognized, but they are considered to extend from most, if not all, neurons in the neocortex. However, when and how cilia develop in neurons are not known. This study used immunohistochemistry for adenylyl cyclase III (ACIII), a marker of primary cilia, and electron microscopic analysis to describe the development and maturation of cilia in mouse neocortical neurons. Our results indicate that ciliogenesis is initiated in late fetal stages after neuroblast migration, when the mother centriole docks with the plasma membrane, becomes a basal body, and grows a cilia bud that we call a procilium. This procilium consists of a membranous protrusion extending from the basal body but lacking axonemal structure and remains undifferentiated until development of the axoneme and cilia elongation starts at about postnatal day 4. Neuronal cilia elongation and final cilia length depend on layer position, and the process extends for a long time, lasting 8-12 weeks. We show that, in addition to pyramidal neurons, inhibitory interneurons also grow cilia of comparable length, suggesting that cilia are indeed present in all neocortical neuron subtypes. Furthermore, the study of mice with defective ciliogenesis suggested that failed elongation of cilia is not essential for proper neuronal migration and laminar organization or establishment of neuronal polarity. Thus, the function of this organelle in neocortical neurons remains elusive.
Copyright © 2011 Wiley Periodicals, Inc.
Figures








References
-
- Anastas SB, Mueller D, Semple-Rowland SL, Breunig JJ, Sarkisian MR. Failed cytokinesis of neural progenitors in citron kinase-deficient rats leads to multiciliated neurons. Cereb Cortex. 2011;21:338–344. (Epub 2010, Jun 4) - PubMed
-
- Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci. 2010;123:62–69. - PubMed
-
- Bearzatto B, Servais L, Roussel C, Gall D, Baba-Aissa F, Schurmans S, de Kerchove d’Exaerde A, Cheron G, Schiffmann SN. Targeted calretinin expression in granule cells of calretinin-null mice restores normal cerebellar functions. FASEB J. 2006;20:380–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

