Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle
- PMID: 22020936
- PMCID: PMC3243543
- DOI: 10.1074/jbc.M111.292755
Junctophilin 1 and 2 proteins interact with the L-type Ca2+ channel dihydropyridine receptors (DHPRs) in skeletal muscle
Abstract
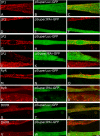
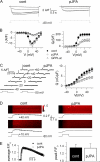
Junctophilins (JPs) anchor the endo/sarcoplasmic reticulum to the plasma membrane, thus contributing to the assembly of junctional membrane complexes in striated muscles and neurons. Recent studies have shown that JPs may be also involved in regulating Ca2+ homeostasis. Here, we report that in skeletal muscle, JP1 and JP2 are part of a complex that, in addition to ryanodine receptor 1 (RyR1), includes caveolin 3 and the dihydropyridine receptor (DHPR). The interaction between JPs and DHPR was mediated by a region encompassing amino acids 230-369 and amino acids 216-399 in JP1 and JP2, respectively. Immunofluorescence studies revealed that the pattern of DHPR and RyR signals in C2C12 cells knocked down for JP1 and JP2 was rather diffused and characterized by smaller puncta in contrast to that observed in control cells. Functional experiments revealed that down-regulation of JPs in differentiated C2C12 cells resulted in a reduction of intramembrane charge movement and the L-type Ca2+ current accompanied by a reduced number of DHPRs at the plasma membrane, whereas there was no substantial alteration in Ca2+ release from the sterol regulatory element-binding protein. Altogether, these results suggest that JP1 and JP2 can facilitate the assembly of DHPR with other proteins of the excitation-contraction coupling machinery.
Figures


References
-
- Franzini-Armstrong C. (2004) in Myology (Engel A. G., Franzini-Armstrong C. eds) pp. 232–256, McGraw-Hill, Inc., New York, NY
-
- Sorrentino V. (2011) Int. J Biochem. Cell Biol. 43, 1075–1078 - PubMed
-
- Rossi A. E., Dirksen R. T. (2006) Muscle Nerve 33, 715–731 - PubMed
-
- Leung A. T, Imagawa T., Campbell K. P. (1987) J. Biol. Chem. 262, 7943–7946 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

