Myotilin dynamics in cardiac and skeletal muscle cells
- PMID: 22021208
- PMCID: PMC3240742
- DOI: 10.1002/cm.20542
Myotilin dynamics in cardiac and skeletal muscle cells
Abstract
Myotilin cDNA has been cloned for the first time from chicken muscles and sequenced. Ectopically expressed chicken and human YFP-myotilin fusion proteins localized in avian muscle cells in the Z-bodies of premyofibrils and the Z-bands of mature myofibrils. Fluorescence recovery after photobleaching experiments demonstrated that chicken and human myotilin were equally dynamic with 100% mobile fraction in premyofibrils and Z-bands of mature myofibrils. Seven myotilin mutants cDNAs (S55F, S55I, T57I, S60C, S60F, S95I, R405K) with known muscular dystrophy association localized in mature myofibrils in the same way as normal myotilin without affecting the formation and maintenance of myofibrils. N- and C-terminal halves of human myotilin were cloned and expressed as YFP fusions in myotubes and cardiomyocytes. N-terminal myotilin (aa 1-250) localized weakly in Z-bands with a high level of unincorporated protein and no adverse effect on myofibril structure. C-terminal myotilin (aa 251-498) localized in Z-bands and in aggregates. Formation of aggregated C-terminal myotilin was accompanied by the loss of Z-band localization of C-terminal myotilin and partial or complete loss of alpha-actinin from the Z-bands. In regions of myotubes with high concentrations of myotilin aggregates there were no alpha-actinin positive Z-bands or organized F-actin. The dynamics of the C-terminal-myotilin and N-terminal myotilin fragments differed significantly from each other and from full-length myotilin. In contrast, no significant changes in dynamics were detected after expression in myotubes of myotilin mutants with single amino acid changes known to be associated with myopathies.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


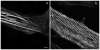


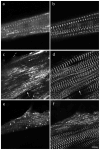

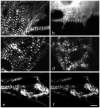


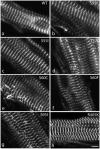

Similar articles
-
An N-terminal fragment of titin coupled to green fluorescent protein localizes to the Z-bands in living muscle cells: overexpression leads to myofibril disassembly.Mol Biol Cell. 1997 Apr;8(4):705-17. doi: 10.1091/mbc.8.4.705. Mol Biol Cell. 1997. PMID: 9247649 Free PMC article.
-
Arg/Abl-binding protein, a Z-body and Z-band protein, binds sarcomeric, costameric, and signaling molecules.Cytoskeleton (Hoboken). 2010 Dec;67(12):808-23. doi: 10.1002/cm.20490. Epub 2010 Nov 10. Cytoskeleton (Hoboken). 2010. PMID: 20886612 Free PMC article.
-
Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells.Cell Motil Cytoskeleton. 2000 Jan;45(1):67-82. doi: 10.1002/(SICI)1097-0169(200001)45:1<67::AID-CM7>3.0.CO;2-T. Cell Motil Cytoskeleton. 2000. PMID: 10618168
-
Myofibrillogenesis in skeletal muscle cells.Clin Orthop Relat Res. 2002 Oct;(403 Suppl):S153-62. doi: 10.1097/00003086-200210001-00018. Clin Orthop Relat Res. 2002. PMID: 12394464 Review.
-
Assembly and Maintenance of Myofibrils in Striated Muscle.Handb Exp Pharmacol. 2017;235:39-75. doi: 10.1007/164_2016_53. Handb Exp Pharmacol. 2017. PMID: 27832381 Review.
Cited by
-
Molecular basis of F-actin regulation and sarcomere assembly via myotilin.PLoS Biol. 2021 Apr 12;19(4):e3001148. doi: 10.1371/journal.pbio.3001148. eCollection 2021 Apr. PLoS Biol. 2021. PMID: 33844684 Free PMC article.
-
Salivary cardiac-enriched FHL2-interacting protein is associated with higher diastolic-to-systolic-blood pressure ratio, sedentary time and center of pressure displacement in healthy 7-9 years old school-children.Front Endocrinol (Lausanne). 2024 Jan 18;15:1292653. doi: 10.3389/fendo.2024.1292653. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38304464 Free PMC article.
-
Jasplakinolide reduces actin and tropomyosin dynamics during myofibrillogenesis.Cytoskeleton (Hoboken). 2014 Sep;71(9):513-29. doi: 10.1002/cm.21189. Epub 2014 Sep 12. Cytoskeleton (Hoboken). 2014. PMID: 25145272 Free PMC article.
-
Nonmuscle myosin II in cardiac and skeletal muscle cells.Cytoskeleton (Hoboken). 2018 Aug;75(8):339-351. doi: 10.1002/cm.21454. Cytoskeleton (Hoboken). 2018. PMID: 29781105 Free PMC article.
-
Deep learning-derived cardiovascular age shares a genetic basis with other cardiac phenotypes.Sci Rep. 2022 Dec 31;12(1):22625. doi: 10.1038/s41598-022-27254-z. Sci Rep. 2022. PMID: 36587059 Free PMC article.
References
-
- Dabiri GA, Ayoob JC, Turnacioglu KK, Sanger JM, Sanger JW. Use of green fluorescent proteins linked to cytoskeletal proteins to analyze myofibrillogenesis in living cells. Meth Enzymol. 1999;302:171–186. - PubMed
-
- Foroud T, Pankratz N, Batchman AP, Pauciulo MW, Vidal R, Miravalle L, Goebel HH, Cushman LJ, Azzarelli B, Horak H, Farlow M, Nichols WC. A mutation in myotilin causes spheroid body myopathy. Neurology. 2005;65:1936–1940. FGre. - PubMed
-
- Moreira ES, Wiltshire TJ, Faulkner G, Nilforoushan A, Vainzof M, Suzuki OT, Valle G, Reeves R, Zatz M, Passos-Bueno MR, Jenne DE. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nature Genetics. 2000;24:163–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

