Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging
- PMID: 22021391
- PMCID: PMC3309870
- DOI: 10.1093/gerona/glr164
Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging
Abstract
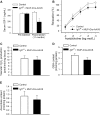
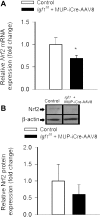
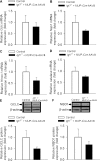
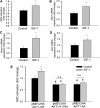
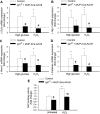
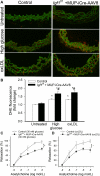
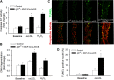
Recent studies demonstrate that age-related dysfunction of NF-E2-related factor-2 (Nrf2)-driven pathways impairs cellular redox homeostasis, exacerbating age-related cellular oxidative stress and increasing sensitivity of aged vessels to oxidative stress-induced cellular damage. Circulating levels of insulin-like growth factor (IGF)-1 decline during aging, which significantly increases the risk for cardiovascular diseases in humans. To test the hypothesis that adult-onset IGF-1 deficiency impairs Nrf2-driven pathways in the vasculature, we utilized a novel mouse model with a liver-specific adeno-associated viral knockdown of the Igf1 gene using Cre-lox technology (Igf1(f/f) + MUP-iCre-AAV8), which exhibits a significant decrease in circulating IGF-1 levels (~50%). In the aortas of IGF-1-deficient mice, there was a trend for decreased expression of Nrf2 and the Nrf2 target genes GCLC, NQO1 and HMOX1. In cultured aorta segments of IGF-1-deficient mice treated with oxidative stressors (high glucose, oxidized low-density lipoprotein, and H(2)O(2)), induction of Nrf2-driven genes was significantly attenuated as compared with control vessels, which was associated with an exacerbation of endothelial dysfunction, increased oxidative stress, and apoptosis, mimicking the aging phenotype. In conclusion, endocrine IGF-1 deficiency is associated with dysregulation of Nrf2-dependent antioxidant responses in the vasculature, which likely promotes an adverse vascular phenotype under pathophysiological conditions associated with oxidative stress (eg, diabetes mellitus, hypertension) and results in accelerated vascular impairments in aging.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

