The extent to which methyl salicylate is required for signaling systemic acquired resistance is dependent on exposure to light after infection
- PMID: 22021417
- PMCID: PMC3327180
- DOI: 10.1104/pp.111.187773
The extent to which methyl salicylate is required for signaling systemic acquired resistance is dependent on exposure to light after infection
Abstract
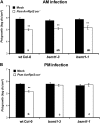
Systemic acquired resistance (SAR) is a state of heightened defense to a broad spectrum of pathogens that is activated throughout a plant following local infection. Development of SAR requires the translocation of one or more mobile signals from the site of infection through the vascular system to distal (systemic) tissues. The first such signal identified was methyl salicylate (MeSA) in tobacco (Nicotiana tabacum). Subsequent studies demonstrated that MeSA also serves as a SAR signal in Arabidopsis (Arabidopsis thaliana) and potato (Solanum tuberosum). By contrast, another study suggested that MeSA is not required for SAR in Arabidopsis and raised questions regarding its signaling role in tobacco. Differences in experimental design, including the developmental age of the plants, the light intensity, and/or the strain of bacterial pathogen, were proposed to explain these conflicting results. Here, we demonstrate that the length of light exposure that plants receive after the primary infection determines the extent to which MeSA is required for SAR signaling. When the primary infection occurred late in the day and as a result infected plants received very little light exposure before entering the night/dark period, MeSA and its metabolizing enzymes were essential for SAR development. In contrast, when infection was done in the morning followed by 3.5 h or more of exposure to light, SAR developed in the absence of MeSA. However, MeSA was generally required for optimal SAR development. In addition to resolving the conflicting results concerning MeSA and SAR, this study underscores the importance of environmental factors on the plant's response to infection.
Figures

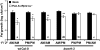
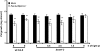
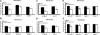


References
-
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18: 1038–1051 - PMC - PubMed
-
- Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, et al. (2011) Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat Genet 43: 421–427 - PubMed
-
- Chandra-Shekara AC, Gupte M, Navarre D, Raina S, Raina R, Klessig D, Kachroo P. (2006) Light-dependent hypersensitive response and resistance signaling against Turnip crinkle virus in Arabidopsis. Plant J 45: 320–334 - PubMed
-
- Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R, Shah J. (2008) Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J 54: 106–117 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

