Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport
- PMID: 22021439
- PMCID: PMC3215072
- DOI: 10.1073/pnas.1108644108
Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport
Abstract
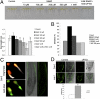
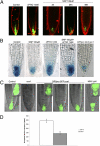
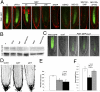
Nitric oxide (NO) is considered a key regulator of plant developmental processes and defense, although the mechanism and direct targets of NO action remain largely unknown. We used phenotypic, cellular, and genetic analyses in Arabidopsis thaliana to explore the role of NO in regulating primary root growth and auxin transport. Treatment with the NO donors S-nitroso-N-acetylpenicillamine, sodium nitroprusside, and S-nitrosoglutathione reduces cell division, affecting the distribution of mitotic cells and meristem size by reducing cell size and number compared with NO depletion by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO). Interestingly, genetic backgrounds in which the endogenous NO levels are enhanced [chlorophyll a/b binding protein underexpressed 1/NO overproducer 1 (cue1/nox1) mirror this response, together with an increased cell differentiation phenotype. Because of the importance of auxin distribution in regulating primary root growth, we analyzed auxin-dependent response after altering NO levels. Both elevated NO supply and the NO-overproducing Arabidopsis mutant cue1/nox1 exhibit reduced expression of the auxin reporter markers DR5pro:GUS/GFP. These effects were accompanied by a reduction in auxin transport in primary roots. NO application and the cue1/nox1 mutation caused decreased PIN-FORMED 1 (PIN1)-GFP fluorescence in a proteasome-independent manner. Remarkably, the cue1/nox1-mutant root phenotypes resemble those of pin1 mutants. The use of both chemical treatments and mutants with altered NO levels demonstrates that high levels of NO reduce auxin transport and response by a PIN1-dependent mechanism, and root meristem activity is reduced concomitantly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wendehenne D, Pugin A, Klessig DF, Durner J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001;6:177–183. - PubMed
-
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. - PubMed
-
- Palmieri MC, et al. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: A bioinformatics approach. J Exp Bot. 2008;59:177–186. - PubMed
-
- Wendehenne D, Durner J, Klessig DF. Nitric oxide: A new player in plant signalling and defense responses. Curr Opin Plant Biol. 2004;7:449–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

