Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin
- PMID: 22021705
- PMCID: PMC3250208
- DOI: 10.1681/ASN.2010111173
Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin
Abstract
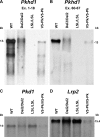
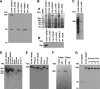
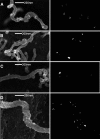
Mutations in the PKHD1 gene, which encodes fibrocystin, cause autosomal recessive polycystic kidney disease (ARPKD). Unfortunately, the lack of specific antibodies to the mouse protein impairs the study of splicing, post-translational processing, shedding, and temporal and spatial expression of endogenous fibrocystin at the cellular and subcellular level. Here, we report using a knock-in strategy to generate a null Pkhd1 strain and a strain that expresses fibrocystin along with two SV5-Pk epitope tags engineered in-frame into the third exon, immediately C-terminal to the signal-peptide cleavage site in a poorly conserved region. By 6 mo of age, the Pkhd1-null mouse develops massive cystic hepatomegaly and proximal tubule dilation, whereas the mouse with epitope-tagged fibrocystin has histologically normal liver and kidneys at 14 mo. Although Pkhd1 was believed to generate many splice forms, our western analysis resolved fibrocystin as a 500 kD product without other forms in the 15-550 kD range. Western analysis also revealed that exosome-like vesicles (ELVs) secrete the bulk of fibrocystin in its mature cleaved form, and scanning electron microscopy identified that fibrocystin on ELVs attached to cilia. Furthermore, the addition of ELVs with epitope-tagged fibrocystin to wild-type cells showed that label transferred to primary cilia within 5 min. In summary, tagging of the endogenous Pkhd1 gene facilitates the study of the glycosylation, proteolytic cleavage, and shedding of fibrocystin.
Figures





Comment in
-
Tagged fibrocystin sheds its secrets.J Am Soc Nephrol. 2011 Dec;22(12):2148-50. doi: 10.1681/ASN.2011101005. Epub 2011 Nov 11. J Am Soc Nephrol. 2011. PMID: 22080423 Free PMC article. No abstract available.
References
-
- Zerres K, Mucher G, Becker J, Steinkamm C, Rudnik-Schoneborn S, Heikkila P, Rapola J, Salonen R, Germino GG, Onuchic L, Somlo S, Avner ED, Harman LA, Stockwin JM, Guay-Woodford LM: Prenatal diagnosis of autosomal recessive polycystic kidney disease (ARPKD): Molecular genetics, clinical experience, and fetal morphology. Am J Med Genet 76: 137–144 1998 - PubMed
-
- Jorgensen MJ: The ductal plate malformation. Acta Pathol Microbiol Scand Suppl: 1–87, 1977 - PubMed
-
- Zerres K, Rudnik-Schoneborn S, Steinkamm C, Becker J, Mucher G: Autosomal recessive poly-cystic kidney disease. J Mol Med 76: 303–309, 1998 - PubMed
-
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

