Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy
- PMID: 22021706
- PMCID: PMC3269929
- DOI: 10.1681/ASN.2010111210
Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy
Abstract
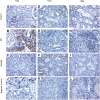
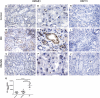
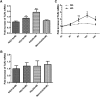
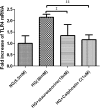
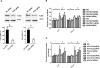
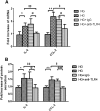
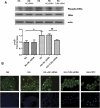
Inflammation contributes to the tubulointerstitial lesions of diabetic nephropathy. Toll-like receptors (TLRs) modulate immune responses and inflammatory diseases, but their role in diabetic nephropathy is not well understood. In this study, we found increased expression of TLR4 but not of TLR2 in the renal tubules of human kidneys with diabetic nephropathy compared with expression of TLR4 and TLR2 in normal kidney and in kidney disease from other causes. The intensity of tubular TLR4 expression correlated directly with interstitial macrophage infiltration and hemoglobin A1c level and inversely with estimated glomerular filtration rate. The tubules also upregulated the endogenous TLR4 ligand high-mobility group box 1 in diabetic nephropathy. In vitro, high glucose induced TLR4 expression via protein kinase C activation in a time- and dose-dependent manner, resulting in upregulation of IL-6 and chemokine (C-C motif) ligand 2 (CCL-2) expression via IκB/NF-κB activation in human proximal tubular epithelial cells. Silencing of TLR4 with small interfering RNA attenuated high glucose-induced IκB/NF-κB activation, inhibited the downstream synthesis of IL-6 and CCL-2, and impaired the ability of conditioned media from high glucose-treated proximal tubule cells to induce transmigration of mononuclear cells. We observed similar effects using a TLR4-neutralizing antibody. Finally, streptozotocin-induced diabetic and uninephrectomized TLR4-deficient mice had significantly less albuminuria, renal dysfunction, renal cortical NF-κB activation, tubular CCL-2 expression, and interstitial macrophage infiltration than wild-type animals. Taken together, these data suggest that a TLR4-mediated pathway may promote tubulointerstitial inflammation in diabetic nephropathy.
Figures

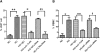


References
-
- Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Nature 414: 782–787, 2001 - PubMed
-
- Navarro-González JF, Mora-Fernández C: The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19: 433–442, 2008 - PubMed
-
- Taft JL, Nolan CJ, Yeung SP, Hewitson TD, Martin FI: Clinical and histological correlations of decline in renal function in diabetic patients with proteinuria. Diabetes 43: 1046–1051, 1994 - PubMed
-
- Mackenzie HS, Ziai F, Omer SA, Nadim MK, Taal MW: Angiotensin receptor blockers in chronic renal disease: the promise of a bright clinical future. J Am Soc Nephrol 10[Suppl 12]: S283–S286, 1999 - PubMed
-
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

