A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee
- PMID: 22022214
- PMCID: PMC3198257
- DOI: 10.7150/ijms.8.615
A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee
Abstract
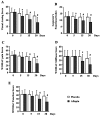
Aflapin(®) is a novel synergistic composition derived from Boswellia serrata gum resin (Indian Patent Application No. 2229/CHE/2008). Aflapin is more efficacious as an anti-inflammatory agent compared to the existing Boswellia products, 5-Loxin(®) and traditional 65% Boswellia extract. A 30-day, double-blind, randomized, placebo-controlled study was conducted to validate the efficacy of Aflapin(®) in the management of clinical symptoms of osteoarthritis (OA) of the knee (Clinical trial registration number: ISRCTN69643551). Sixty eligible OA subjects selected through screening were included in the study. The subjects received either 100 mg (n=30) of Aflapin(®) or placebo (n=30) daily for 30 days. Each subject was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne's Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 5, 15 and 30. A series of biochemical tests in serum, urine and hematological parameters established the safety of Aflapin. The observations suggest that Aflapin conferred clinically and statistically significant improvements in pain scores and physical function scores in OA subjects. Aflapin provided significant improvements in pain score and functional ability in as early as 5 days of treatment. In conclusion, our observations suggest that Aflapin is a safe, fast acting and effective alternative intervention in the management of OA.
Keywords: Aflapin; Boswellia serrata; Clinical study; Osteoarthritis; Visual Analog Scale.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures

References
-
- Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1–9. - PubMed
-
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. - PubMed
-
- Bos SD, Slagboon PE, Meulenbelt I. New insights into osteoarthritis: early developmental features of an aging related disease. Curr Opin Rheumatol. 2008;20:553–559. - PubMed
-
- Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, Cluzeau F, Cooper C, Dieppe PA, Günther KP, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis PM, Leeb B, Lequesne M, Lohmander S, Mazieres B, Mola EM, Pavelka K, Serni U, Swoboda B, Verbruggen AA, Weseloh G, Zimmermann-Gorska I. EULAR recommendations for the management of osteoarthritis: report of task force standing committee for International Clinical Studies including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2000;59:936–944. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

