A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression
- PMID: 22022268
- PMCID: PMC3192849
- DOI: 10.1371/journal.ppat.1002300
A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression
Abstract
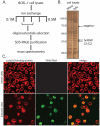
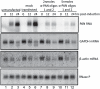
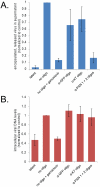
During the lytic phase of infection, the gamma herpesvirus Kaposi's Sarcoma-Associated Herpesvirus (KSHV) expresses a highly abundant, 1.1 kb nuclear noncoding RNA of unknown function. We observe that this polyadenylated nuclear (PAN) RNA avidly binds host poly(A)-binding protein C1 (PABPC1), which normally functions in the cytoplasm to bind the poly(A) tails of mRNAs, regulating mRNA stability and translation efficiency. During the lytic phase of KSHV infection, PABPC1 is re-localized to the nucleus as a consequence of expression of the viral shutoff exonuclease (SOX) protein; SOX also mediates the host shutoff effect in which host mRNAs are downregulated while viral mRNAs are selectively expressed. We show that whereas PAN RNA is not required for the host shutoff effect or for PABPC1 re-localization, SOX strongly upregulates the levels of PAN RNA in transient transfection experiments. This upregulation is destroyed by the same SOX mutation that ablates the host shutoff effect and PABPC1 nuclear re-localization or by removal of the poly(A) tail of PAN. In cells induced into the KSHV lytic phase, depletion of PAN RNA using RNase H-targeting antisense oligonucleotides reveals that it is necessary for the production of late viral proteins from mRNAs that are themselves polyadenylated. Our results add to the repertoire of functions ascribed to long noncoding RNAs and suggest a mechanism of action for nuclear noncoding RNAs in gamma herpesvirus infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Kaposi's Sarcoma-Associated Herpesvirus mRNA Accumulation in Nuclear Foci Is Influenced by Viral DNA Replication and Viral Noncoding Polyadenylated Nuclear RNA.J Virol. 2018 Jun 13;92(13):e00220-18. doi: 10.1128/JVI.00220-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29643239 Free PMC article.
-
Stability of a long noncoding viral RNA depends on a 9-nt core element at the RNA 5' end to interact with viral ORF57 and cellular PABPC1.Int J Biol Sci. 2011;7(8):1145-60. doi: 10.7150/ijbs.7.1145. Epub 2011 Oct 16. Int J Biol Sci. 2011. PMID: 22043172 Free PMC article.
-
Interplay between polyadenylate-binding protein 1 and Kaposi's sarcoma-associated herpesvirus ORF57 in accumulation of polyadenylated nuclear RNA, a viral long noncoding RNA.J Virol. 2013 Jan;87(1):243-56. doi: 10.1128/JVI.01693-12. Epub 2012 Oct 17. J Virol. 2013. PMID: 23077296 Free PMC article.
-
New insights into the expression and functions of the Kaposi's sarcoma-associated herpesvirus long noncoding PAN RNA.Virus Res. 2016 Jan 2;212:53-63. doi: 10.1016/j.virusres.2015.06.012. Epub 2015 Jun 21. Virus Res. 2016. PMID: 26103097 Free PMC article. Review.
-
PAN's Labyrinth: Molecular biology of Kaposi's sarcoma-associated herpesvirus (KSHV) PAN RNA, a multifunctional long noncoding RNA.Viruses. 2014 Nov 4;6(11):4212-26. doi: 10.3390/v6114212. Viruses. 2014. PMID: 25375885 Free PMC article. Review.
Cited by
-
Long noncoding RNAs in innate immunity.Cell Mol Immunol. 2016 Mar;13(2):138-47. doi: 10.1038/cmi.2015.68. Epub 2015 Aug 17. Cell Mol Immunol. 2016. PMID: 26277893 Free PMC article. Review.
-
A lytic viral long noncoding RNA modulates the function of a latent protein.J Virol. 2014 Feb;88(3):1843-8. doi: 10.1128/JVI.03251-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257619 Free PMC article.
-
G3BP1, G3BP2 and CAPRIN1 are required for translation of interferon stimulated mRNAs and are targeted by a dengue virus non-coding RNA.PLoS Pathog. 2014 Jul 3;10(7):e1004242. doi: 10.1371/journal.ppat.1004242. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 24992036 Free PMC article.
-
Idiosyncrasies of Viral Noncoding RNAs Provide Insights into Host Cell Biology.Annu Rev Virol. 2019 Sep 29;6(1):297-317. doi: 10.1146/annurev-virology-092818-015811. Epub 2019 Apr 30. Annu Rev Virol. 2019. PMID: 31039329 Free PMC article. Review.
-
Human Cytomegalovirus RNA2.7 Is Required for Upregulating Multiple Cellular Genes To Promote Cell Motility and Viral Spread Late in Lytic Infection.J Virol. 2021 Sep 27;95(20):e0069821. doi: 10.1128/JVI.00698-21. Epub 2021 Aug 4. J Virol. 2021. PMID: 34346763 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

