Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium
- PMID: 22022271
- PMCID: PMC3192845
- DOI: 10.1371/journal.ppat.1002319
Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium
Abstract
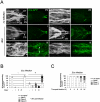
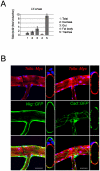
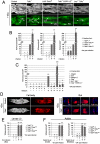
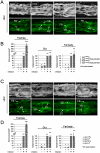
Barrier epithelia that are persistently exposed to microbes have evolved potent immune tools to eliminate such pathogens. If mechanisms that control Drosophila systemic responses are well-characterized, the epithelial immune responses remain poorly understood. Here, we performed a genetic dissection of the cascades activated during the immune response of the Drosophila airway epithelium i.e. trachea. We present evidence that bacteria induced-antimicrobial peptide (AMP) production in the trachea is controlled by two signalling cascades. AMP gene transcription is activated by the inducible IMD pathway that acts non-cell autonomously in trachea. This IMD-dependent AMP activation is antagonized by a constitutively active signalling module involving the receptor Toll-8/Tollo, the ligand Spätzle2/DNT1 and Ect-4, the Drosophila ortholog of the human Sterile alpha and HEAT/ARMadillo motif (SARM). Our data show that, in addition to Toll-1 whose function is essential during the systemic immune response, Drosophila relies on another Toll family member to control the immune response in the respiratory epithelium.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
-
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. - PubMed
-
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

