Binge Drinking: In Search of its Molecular Target via the GABA(A) Receptor
- PMID: 22022305
- PMCID: PMC3195989
- DOI: 10.3389/fnins.2011.00123
Binge Drinking: In Search of its Molecular Target via the GABA(A) Receptor
Abstract
Binge drinking, frequently referred to clinically as problem or hazardous drinking, is a pattern of excessive alcohol intake characterized by blood alcohol levels ≥0.08 g% within a 2-h period. Here, we show that overexpression of α1 subunits of the GABA(A) receptor contributes to binge drinking, and further document that this involvement is related to the neuroanatomical localization of α1 receptor subunits. Using a herpes simplex virus amplicon vector to deliver small interference RNA (siRNA), we showed that siRNA specific for the α1 subunit (pHSVsiLA1) caused profound, long-term, and selective reduction of gene expression, receptor density, and binge drinking in high-alcohol drinking rats when delivered into the ventral pallidum (VP). Scrambled siRNA (pHSVsiNC) delivered similarly into the VP failed to alter gene expression, receptor density, or binge drinking. Silencing of the α1 gene in the VP, however, failed to alter binge sucrose or water intake. These results, along with our prior research, provide compelling evidence that the α1-containing GABA(A) receptor subunits are critical in the regulation of binge-like patterns of excessive drinking. Collectively, these data may be useful in the development of gene-based and novel pharmacological approaches for the treatment of excessive drinking.
Keywords: GABAA receptor; HAD rat; alcohol; alpha 1; binge drinking; siRNA; ventral pallidum; viral vector.
Figures






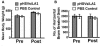
References
-
- Boehm S. L., II, Ponomarev I., Jennings A. W., Whiting P. J., Rosahl T. W., Garrett E. M., Blednov Y. A., Harris R. A. (2004). Gamma-aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem. Pharmacol. 68, 1581–1602 [Review].10.1016/j.bcp.2004.07.023 - DOI - PubMed
-
- Center for Disease Control and Prevention (CDCP) (2004). Alcohol-attributable deaths and years of potential life lost – United States 2001. MMWR Morb. Mortal. Wkly. Rep. 53, 866–870 - PubMed
-
- Chikritzhs T. N., Jonas H. A., Stockwell T. R., Heale P. F., Dietze P. M. (2001). Mortality and life-years lost due to alcohol: a comparison of acute and chronic causes. Med. J. Aust. 174, 281–284 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

