Epoetin Theta with a New Dosing Schedule in Anaemic Cancer Patients Receiving Nonplatinum-Based Chemotherapy: A Randomised Controlled Trial
- PMID: 22022341
- PMCID: PMC3193379
- DOI: 10.1111/j.1753-5174.2011.00035.x
Epoetin Theta with a New Dosing Schedule in Anaemic Cancer Patients Receiving Nonplatinum-Based Chemotherapy: A Randomised Controlled Trial
Abstract
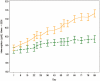
INTRODUCTION: Recombinant human erythropoietin (r-HuEPO) is used to treat symptomatic anaemia due to chemotherapy. A new r-HuEPO, Epoetin theta (Eporatio®), was investigated and compared to placebo in a randomised, double-blind clinical trial in adult cancer patients receiving nonplatinum-based chemotherapy. The primary efficacy endpoint was the responder rate (complete haemoglobin (Hb) response, i.e., Hb increase ≥2 g/dl) without the benefit of a transfusion within the previous 4 weeks. RESEARCH DESIGN AND METHODS: 186 patients were randomised to s.c. treatment for 12 weeks with either Epoetin theta (N = 95) or placebo (N = 91). The starting dose was 20,000 IU once weekly Epoetin theta or placebo. RESULTS: The incidence of complete Hb responders was significantly higher in the Epoetin theta group than in the placebo group (72.6 vs. 25.3%, P < 0.0001). More patients in the placebo group than in the Epoetin theta group received blood transfusions after randomisation (23 patients, 25.3% vs. 13 patients, 13.7%, P = 0.0277). The majority of patients with a complete Hb response had 20,000 IU/week as their maximum dose prior to response, indicating that a dose of 20,000 IU is an appropriate starting dose. The overall frequencies of adverse events (AEs) were similar in both treatment groups. Hypertension was the only AE that was more frequent in the Epoetin theta group compared to the placebo group (8.4 vs. 1.1%). CONCLUSIONS: Epoetin theta showed a superior efficacy to placebo in terms of complete Hb response without blood transfusion within the previous 4 weeks. Treatment with Epoetin theta resulted in a statistically significant increase in mean haemoglobin levels compared to placebo. The overall frequencies of adverse events were similar in both treatment groups.
Figures
References
-
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin: Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73–8. - PubMed
-
- Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175–8. - PubMed
-
- Cazzola M, Messinger D, Battistel V, Bron D, Cimino R, Enller-Ziegler L, et al. Recombinant human erythropoietin in the anemia associated with multiple myeloma or non-Hodgkin's lymphoma: Dose finding and identification of predictors of response. Blood. 1995;86:4446–53. - PubMed
-
- Cazzola M, Beguin Y, Kloczko J, Spicka I, Coiffier B. Once-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. Br J Haematol. 2003;122:386–93. - PubMed
-
- Granetto C, Ricci S, Martoni A, Pezzella G, Testore F, Mattioli R, et al. Comparing the efficacy and safety of fixed versus weight-based dosing of epoetin alpha in anemic cancer patients receiving platinum-based chemotherapy. Oncol Rep. 2003;10:1289–96. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources