Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells
- PMID: 22022444
- PMCID: PMC3192723
- DOI: 10.1371/journal.pone.0025761
Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells
Abstract
We have previously shown that human embryonic stem cells can be differentiated into embryonic and fetal type of red blood cells that sequentially express three types of hemoglobins recapitulating early human erythropoiesis. We report here that we have produced iPS from three somatic cell types: adult skin fibroblasts as well as embryonic and fetal mesenchymal stem cells. We show that regardless of the age of the donor cells, the iPS produced are fully reprogrammed into a pluripotent state that is undistinguishable from that of hESCs by low and high-throughput expression and detailed analysis of globin expression patterns by HPLC. This suggests that reprogramming with the four original Yamanaka pluripotency factors leads to complete erasure of all functionally important epigenetic marks associated with erythroid differentiation regardless of the age or the tissue type of the donor cells, at least as detected in these assays. The ability to produce large number of erythroid cells with embryonic and fetal-like characteristics is likely to have many translational applications.
Conflict of interest statement
Figures
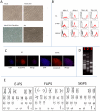
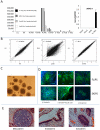

References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. - PubMed
-
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

