Age-related impairment of ultrasonic vocalization in Tau.P301L mice: possible implication for progressive language disorders
- PMID: 22022446
- PMCID: PMC3192129
- DOI: 10.1371/journal.pone.0025770
Age-related impairment of ultrasonic vocalization in Tau.P301L mice: possible implication for progressive language disorders
Abstract
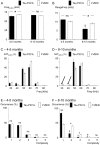
Background: Tauopathies, including Alzheimer's Disease, are the most frequent neurodegenerative diseases in elderly people and cause various cognitive, behavioural and motor defects, but also progressive language disorders. For communication and social interactions, mice produce ultrasonic vocalization (USV) via expiratory airflow through the larynx. We examined USV of Tau.P301L mice, a mouse model for tauopathy expressing human mutant tau protein and developing cognitive, motor and upper airway defects.
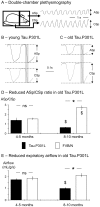
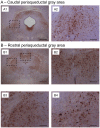
Methodology/principal findings: At age 4-5 months, Tau.P301L mice had normal USV, normal expiratory airflow and no brainstem tauopathy. At age 8-10 months, Tau.P301L mice presented impaired USV, reduced expiratory airflow and severe tauopathy in the periaqueductal gray, Kolliker-Fuse and retroambiguus nuclei. Tauopathy in these nuclei that control upper airway function and vocalization correlates well with the USV impairment of old Tau.P301L mice.
Conclusions: In a mouse model for tauopathy, we report for the first time an age-related impairment of USV that correlates with tauopathy in midbrain and brainstem areas controlling vocalization. The vocalization disorder of old Tau.P301L mice could be, at least in part, reminiscent of language disorders of elderly suffering tauopathy.
Conflict of interest statement
Figures



References
-
- Humbert IA, McLaren DG, Kosmatka K, Fitzgerald M, Johnson S, et al. Early deficits in cortical control of swallowing in Alzheimer's disease. J Alzheimers Dis. 2010;19:1185–1197. doi: 10.3233/JAD-2010-1316. - DOI - PMC - PubMed
-
- Suh MK, Kim H, Na DL. Dysphagia in patients with dementia: Alzheimer versus vascular. Alzheimer Dis Assoc Disord. 2009;23:178–184. doi: 10.1097/WAD.0b013e318192a539. - DOI - PubMed
-
- Attems J, König C, Huber M, Lintner F, Jellinger KA. Cause of death in demented and non-demented elderly inpatients; an autopsy study of 308 cases. J Alzheimers Dis. 2005;8:57–62. - PubMed
-
- Onen F, Onen H. [Obstructive sleep apnea and cognitive impairment in the elderly]. Psychol Neuropsychiatr Vieil. 2010;8:163–169. doi: 10.1684/pnv.2010.0219. - DOI - PubMed
-
- Cooke JR, Liu L, Natarajan L, He F, Marler M, et al. The effect of sleep-disordered breathing on stages of sleep in patients with Alzheimer's disease. Behav Sleep Med. 2006;4:219–27. doi: 10.1207/s15402010bsm0404_2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

