Bioluminescent orthotopic mouse models of human localized non-small cell lung cancer: feasibility and identification of circulating tumour cells
- PMID: 22022511
- PMCID: PMC3191172
- DOI: 10.1371/journal.pone.0026073
Bioluminescent orthotopic mouse models of human localized non-small cell lung cancer: feasibility and identification of circulating tumour cells
Abstract
Background: Preclinical models of non-small cell lung cancer (NSCLC) require better clinical relevance to study disease mechanisms and innovative therapeutics. We sought to compare and refine bioluminescent orthotopic mouse models of human localized NSCLC.
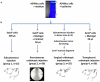
Methods: Athymic nude mice underwent subcutaneous injection (group 1-SC, n = 15, control), percutaneous orthotopic injection (group 2-POI, n = 30), surgical orthotopic implantation of subcutaneously grown tumours (group 3-SOI, n = 25), or transpleural orthotopic injection (group 4-TOI, n = 30) of A549-luciferase cells. Bioluminescent in vivo imaging was then performed weekly. Circulating tumour cells (CTCs) were searched using Cellsearch® system in SC and TOI models.
Results: Group 2-POI was associated with unexpected direct pleural spreading of the cellular solution in 53% of the cases, forbidding further evaluation of any localized lung tumour. Group 3-SOI was characterized by high perioperative mortality, initially localized lung tumours, and local evolution. Group 4-TOI was associated with low perioperative mortality, initially localized lung tumours, loco regional extension, and distant metastasis. CTCs were detected in 83% of nude mice bearing subcutaneous or orthotopic NSCLC tumours.
Conclusions: Transpleural orthotopic injection of A549-luc cells in nude mouse lung induces localized tumour, followed by lymphatic extension and specific mortality, and allowed the first time identification of CTCs in a NSCLC mice model.
Conflict of interest statement
Figures



References
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. - PubMed
-
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (iressa survival evaluation in lung cancer). Lancet. 2005;366:1527–37. - PubMed
-
- Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9. - PubMed
-
- Soria JC, Blay JY, Spano JP, Pivot X, Coscas Y, et al. Added value of molecular targeted agents in oncology. Ann Oncol. 2011;22:1703–16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

