Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells
- PMID: 22022533
- PMCID: PMC3192146
- DOI: 10.1371/journal.pone.0026125
Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells
Abstract
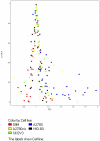
Thrombosis is common in ovarian cancer. However, the interaction of platelets with ovarian cancer cells has not been critically examined. To address this, we investigated platelet interactions in a range of ovarian cancer cell lines with different metastatic potentials [HIO-80, 59M, SK-OV-3, A2780, A2780cis]. Platelets adhered to ovarian cancer cells with the most significant adhesion to the 59M cell line. Ovarian cancer cells induced platelet activation [P-selectin expression] in a dose dependent manner, with the most significant activation seen in response to the 59M cell line. The platelet antagonists [cangrelor, MRS2179, and apyrase] inhibited 59M cell induced activation suggesting a P2Y12 and P2Y1 receptor mediated mechanism of platelet activation dependent on the release of ADP by 59M cells. A2780 and 59M cells potentiated PAR-1, PAR-4, and TxA2 receptor mediated platelet activation, but had no effect on ADP, epinephrine, or collagen induced activation. Analysis of gene expression changes in ovarian cancer cells following treatment with washed platelets or platelet releasate showed a subtle but valid upregulation of anti-apoptotic, anti-autophagy pro-angiogenic, pro-cell cycle and metabolic genes. Thus, ovarian cancer cells with different metastatic potential adhere and activate platelets differentially while both platelets and platelet releasate mediate pro-survival and pro-angiogenic signals in ovarian cancer cells.
Conflict of interest statement
Figures



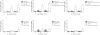


References
-
- Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26:995–1005. - PubMed
-
- Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. - PubMed
-
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. - PubMed
-
- Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Stoler MH, et al. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19:57–63. - PubMed
-
- Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, et al. Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. J Clin Oncol. 2001;19:368–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

