Dtorsin, the Drosophila ortholog of the early-onset dystonia TOR1A (DYT1), plays a novel role in dopamine metabolism
- PMID: 22022556
- PMCID: PMC3192163
- DOI: 10.1371/journal.pone.0026183
Dtorsin, the Drosophila ortholog of the early-onset dystonia TOR1A (DYT1), plays a novel role in dopamine metabolism
Erratum in
- PLoS One. 2011;6(10). doi:10.1371/annotation/b76ab6b8-32c7-4f47-9ec9-9f302c9507f9
Abstract
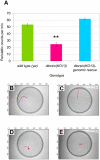
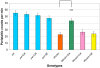
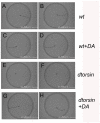
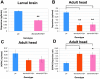
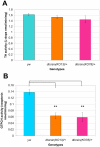
Dystonia represents the third most common movement disorder in humans. At least 15 genetic loci (DYT1-15) have been identified and some of these genes have been cloned. TOR1A (formally DYT1), the gene responsible for the most common primary hereditary dystonia, encodes torsinA, an AAA ATPase family protein. However, the function of torsinA has yet to be fully understood. Here, we have generated and characterized a complete loss-of-function mutant for dtorsin, the only Drosophila ortholog of TOR1A. Null mutation of the X-linked dtorsin was semi-lethal with most male flies dying by the pre-pupal stage and the few surviving adults being sterile and slow moving, with reduced cuticle pigmentation and thin, short bristles. Third instar male larvae exhibited locomotion defects that were rescued by feeding dopamine. Moreover, biochemical analysis revealed that the brains of third instar larvae and adults heterozygous for the loss-of-function dtorsin mutation had significantly reduced dopamine levels. The dtorsin mutant showed a very strong genetic interaction with Pu (Punch: GTP cyclohydrolase), the ortholog of the human gene underlying DYT14 dystonia. Biochemical analyses revealed a severe reduction of GTP cyclohydrolase protein and activity, suggesting that dtorsin plays a novel role in dopamine metabolism as a positive-regulator of GTP cyclohydrolase protein. This dtorsin mutant line will be valuable for understanding this relationship and potentially other novel torsin functions that could play a role in human dystonia.
Conflict of interest statement
Figures




References
-
- Bruggemann N, Klein C. Genetics of primary torsion dystonia. Curr Neurol Neurosci Rep. 2010;10:199–206. - PubMed
-
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. - PubMed
-
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. - PubMed
-
- Ozelius LJ, Page CE, Klein C, Hewett JW, Mineta M, et al. The TOR1A (DYT1) gene family and its role in early onset torsion dystonia. Genomics. 1999;62:377–384. - PubMed
-
- Kock N, Naismith TV, Boston HE, Ozelius LJ, Corey DP, et al. Effects of genetic variations in the dystonia protein torsinA: identification of polymorphism at residue 216 as protein modifier. Hum Mol Genet. 2006;15:1355–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

