Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study
- PMID: 22023513
- PMCID: PMC3618489
- DOI: 10.1111/j.1537-2995.2011.03401.x
Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study
Abstract
Background: Blood donors are at risk of iron deficiency. We evaluated the effects of blood donation intensity on iron and hemoglobin (Hb) in a prospective study.
Study design and methods: Four cohorts of frequent and first-time or reactivated (FT/RA) blood donors (no donation in 2 years), female and male, totaling 2425, were characterized and followed as they donated blood frequently. At enrollment and the final visit, ferritin, soluble transferrin receptor (sTfR), and Hb were determined. Models to predict iron deficiency and Hb deferral were developed. Iron depletion was defined at two levels: iron deficiency erythropoiesis (IDE) [log(sTfR/ferritin) ≥ 2.07] and absent iron stores (AIS; ferritin < 12 ng/mL).
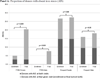
Results: Among returning female FT and RA donors, 20 and 51% had AIS and IDE at their final visit, respectively; corresponding proportions for males were 8 and 20%. Among female frequent donors who returned, 27 and 62% had AIS and IDE, respectively, while corresponding proportions for males were 18 and 47%. Predictors of IDE and/or AIS included a higher frequency of blood donation in the past 2 years, a shorter interdonation interval, and being female and young; conversely, taking iron supplements reduced the risk of iron depletion. Predictors of Hb deferral included female sex, black race, and a shorter interdonation interval.
Conclusions: There is a high prevalence of iron depletion in frequent blood donors. Increasing the interdonation interval would reduce the prevalence of iron depletion and Hb deferral. Alternatively, replacement with iron supplements may allow frequent donation without the adverse outcome of iron depletion.
© 2011 American Association of Blood Banks.
Conflict of interest statement
The remaining authors declare that they have no conflicts of interest relevant to the manuscript.
Figures




Comment in
-
The REDS-II Donor Iron Status Evaluation (RISE) study: conclusions should not exceed the limitations of the data.Transfusion. 2012 Jun;52(6):1382-3; author reply 1383-5. doi: 10.1111/j.1537-2995.2012.03653.x. Transfusion. 2012. PMID: 22686535 No abstract available.
References
-
- Simon TL, Garry PJ, Hooper EM. Iron stores in blood donors. JAMA. 1981;245(20):2038–2043. - PubMed
-
- Mast AE, Foster TM, Pinder HL, Beczkiewicz CA, Bellissimo DB, Murphy AT, et al. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48(10):2197. - PubMed
-
- Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood. 1977;50(3):441–447. - PubMed
-
- Newman B. Iron depletion by whole-blood donation harms menstruating females: The current whole-blood-collection paradigm needs to be changed. Transfusion. 2006;46(10):1667–1681. - PubMed
-
- Sullivan JL. Stored iron and vascular reactivity. Arterioscler Thromb Vasc Biol. 2005;25(8):1532–1535. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

