Subunit III-depleted cytochrome c oxidase provides insight into the process of proton uptake by proteins
- PMID: 22023935
- PMCID: PMC3294125
- DOI: 10.1016/j.bbabio.2011.10.001
Subunit III-depleted cytochrome c oxidase provides insight into the process of proton uptake by proteins
Abstract
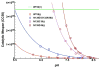
We review studies of subunit III-depleted cytochrome c oxidase (CcO III (-)) that elucidate the structural basis of steady-state proton uptake from solvent into an internal proton transfer pathway. The removal of subunit III from R. sphaeroides CcO makes proton uptake into the D pathway a rate-determining step, such that measurements of the pH dependence of steady-state O(2) consumption can be used to compare the rate and functional pK(a) of proton uptake by D pathways containing different initial proton acceptors. The removal of subunit III also promotes spontaneous suicide inactivation by CcO, greatly shortening its catalytic lifespan. Because the probability of suicide inactivation is controlled by the rate at which the D pathway delivers protons to the active site, measurements of catalytic lifespan provide a second method to compare the relative efficacy of proton uptake by engineered CcO III (-) forms. These simple experimental systems have been used to explore general questions of proton uptake by proteins, such as the functional value of an initial proton acceptor, whether an initial acceptor must be surface-exposed, which side chains will function as initial proton acceptors and whether multiple acceptors can speed proton uptake.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




References
-
- Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
-
- Wraight CA. Chance and design--proton transfer in water, channels and bioenergetic proteins. Biochim Biophys Acta. 2006;1757:886–912. - PubMed
-
- Brzezinski P, Ädelroth P. Design principles of proton-pumping haem-copper oxidases. Curr Opin Struct Biol. 2006;16:465–472. - PubMed
-
- Brändén G, Gennis RB, Brzezinski P. Transmembrane proton translocation by cytochrome c oxidase. Biochim Biophys Acta. 2006;1757:1052–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

