Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder
- PMID: 22024001
- PMCID: PMC3225067
- DOI: 10.1016/j.jaac.2011.08.002
Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder
Abstract
Objective: Because of significant individual variability in attention-deficit/hyperactivity disorder (ADHD) medication response, there is increasing interest in identifying genetic predictors of treatment effects. This study examined the role of four catecholamine-related candidate genes in moderating methylphenidate (MPH) dose-response.
Method: Eighty-nine stimulant-naive children with ADHD 7 to 11 years old participated in a randomized, double-blind, crossover trial of long-acting MPH. Parents and teachers assessed each child's response on placebo and three MPH dosage levels using the Vanderbilt ADHD rating scales. Children were genotyped for polymorphisms in the 3' untranslated region of dopamine transporter (DAT), exon 3 on dopamine receptor D(4) (DRD4), codon 158 on catechol-O-methyltransferase, and the adrenergic α(2A)-receptor promoter. Linear mixed models evaluated gene, dose (milligrams per kilogram per day), and gene-by-dose effects on inattentive and hyperactive-impulsive domain outcomes.
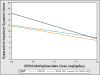
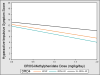
Results: The most statistically significant gene-by-dose interactions were observed on hyperactive-impulsive symptoms for DRD4 and DAT polymorphisms, with participants lacking the DAT 10-repeat allele showing greater improvements in symptoms with increasing dose compared with 10-repeat carriers (p = .008) and those lacking the DRD4 4-repeat allele showing less improvement across MPH doses compared with 4-repeat carriers (p = 0.02).
Conclusions: This study suggests that DAT and DRD4 polymorphisms may be associated with individual variability in MPH dose-response, although further research in larger samples is required to confirm these findings and their clinical utility.
Clinical trial registration information: Response Variability in Children with Attention-Deficit/Hyperactivity Disorder (ADHD); http://www.clinicaltrials.gov; NCT01238822.
Copyright © 2011 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001 Oct;108(4):1033–1044. - PubMed
-
- Greenhill LL, Swanson JM, Vitiello B, et al. Impairment and deportment responses to different methylphenidate doses in children with ADHD: the MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001 Feb;40(2):180–187. - PubMed
-
- Wolraich ML, Doffing MA. Pharmacokinetic considerations in the treatment of attention-deficit hyperactivity disorder with methylphenidate. CNS drugs. 2004;18(4):243–250. - PubMed
-
- Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S. Methylphenidate in hyperactive children: differential effects of dose on academic, learning, and social behavior. Journal of abnormal child psychology. 1985 Jun;13(2):227–243. - PubMed
-
- Lowe N, Barry E, Gill M, Hawi Z. An Overview of the Pharmacogenetics and Molecular Genetics of ADHD. Current Pharmacogenomics. 2006;4:231–243.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

