Role of tumor suppressor p53 in megakaryopoiesis and platelet function
- PMID: 22024107
- PMCID: PMC3258354
- DOI: 10.1016/j.exphem.2011.10.006
Role of tumor suppressor p53 in megakaryopoiesis and platelet function
Abstract
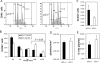
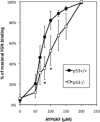
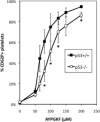
The pathobiological role of p53 has been widely studied, however, its role in normophysiology is relatively unexplored. We previously showed that p53 knock-down increased ploidy in megakaryocytic cultures. This study aims to examine the effect of p53 loss on in vivo megakaryopoiesis, platelet production, and function, and to investigate the basis for greater ploidy in p53(-/-) megakaryocytic cultures. Here, we used flow cytometry to analyze ploidy, DNA synthesis, and apoptosis in murine cultured and bone marrow megakaryocytes following thrombopoietin administration and to analyze fibrinogen binding to platelets in vitro. Culture of p53(-/-) marrow cells for 6 days with thrombopoietin gave rise to 1.7-fold more megakaryocytes, 26.1% ± 3.6% of which reached ploidy classes ≥64 N compared to 8.2% ± 0.9% of p53(+/+) megakaryocytes. This was due to 30% greater DNA synthesis in p53(-/-) megakaryocytes and 31% greater apoptosis in p53(+/+) megakaryocytes by day 4 of culture. Although the bone marrow and spleen steady-state megakaryocytic content and ploidy were similar in p53(+/+) and p53(-/-) mice, thrombopoietin administration resulted in increased megakaryocytic polyploidization in p53(-/-) mice. Although their platelet counts were normal, p53(-/-) mice exhibited significantly longer bleeding times and p53(-/-) platelets were less sensitive than p53(+/+) platelets to agonist-induced fibrinogen binding and P-selectin secretion. In summary, our in vivo and ex vivo studies indicate that p53 loss leads to increased polyploidization during megakaryopoiesis. Our findings also suggest for the first time a direct link between p53 loss and the development of fully functional platelets resulting in hemostatic deficiencies.
Copyright © 2012 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures

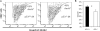


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

