The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a
- PMID: 22024412
- PMCID: PMC3268837
- DOI: 10.1016/j.expneurol.2011.10.005
The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a
Abstract
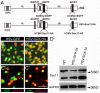
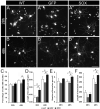
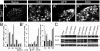
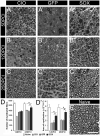
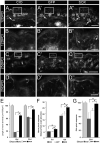
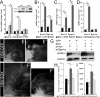
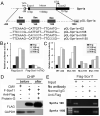
Factors that enhance the intrinsic growth potential of adult neurons are key players in the successful repair and regeneration of neurons following injury. Injury-induced activation of transcription factors has a central role in this process because they regulate expression of regeneration-associated genes. Sox11 is a developmentally expressed transcription factor that is significantly induced in adult neurons in response to injury. Its function in injured neurons is however undefined. Here, we report studies that use herpes simplex virus (HSV)-vector-mediated expression of Sox11 in adult sensory neurons to assess the effect of Sox11 overexpression on neuron regeneration. Cultured mouse dorsal root ganglia (DRG) neurons transfected with HSV-Sox11 exhibited increased neurite elongation and branching relative to naïve and HSV-vector control treated neurons. Neurons from mice injected in foot skin with HSV-Sox11 exhibited accelerated regeneration of crushed saphenous nerves as indicated by faster regrowth of axons and nerve fibers to the skin, increased myelin thickness and faster return of nerve and skin sensitivity. Downstream targets of HSV-Sox11 were examined by analyzing changes in gene expression of known regeneration-associated genes. This analysis in combination with mutational and chromatin immunoprecipitation assays indicates that the ability of Sox11 to accelerate in vivo nerve regeneration is dependent on its transcriptional activation of the regeneration-associated gene, small proline rich protein 1a (Sprr1a). This finding reveals a new functional linkage between Sox11 and Sprr1a in adult peripheral neuron regeneration.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Expression of the regeneration-associated protein SPRR1A in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury.J Comp Neurol. 2009 Mar 1;513(1):51-68. doi: 10.1002/cne.21944. J Comp Neurol. 2009. PMID: 19107756 Free PMC article.
-
Sox11 transcription factor modulates peripheral nerve regeneration in adult mice.Brain Res. 2009 Feb 23;1256:43-54. doi: 10.1016/j.brainres.2008.12.032. Epub 2008 Dec 24. Brain Res. 2009. PMID: 19133245 Free PMC article.
-
TRAF family member-associated NF-kappa B activator (TANK) expression increases in injured sensory neurons and is transcriptionally regulated by Sox11.Neuroscience. 2013 Feb 12;231:28-37. doi: 10.1016/j.neuroscience.2012.11.034. Epub 2012 Nov 29. Neuroscience. 2013. PMID: 23201825 Free PMC article.
-
Expression of Regeneration-Associated Proteins in Primary Sensory Neurons and Regenerating Axons After Nerve Injury-An Overview.Anat Rec (Hoboken). 2018 Oct;301(10):1618-1627. doi: 10.1002/ar.23843. Epub 2018 May 13. Anat Rec (Hoboken). 2018. PMID: 29740961 Review.
-
Transcriptional Control of Peripheral Nerve Regeneration.Mol Neurobiol. 2023 Jan;60(1):329-341. doi: 10.1007/s12035-022-03090-0. Epub 2022 Oct 20. Mol Neurobiol. 2023. PMID: 36261692 Review.
Cited by
-
Sox11 is enriched in myogenic progenitors but dispensable for development and regeneration of the skeletal muscle.Skelet Muscle. 2023 Sep 13;13(1):15. doi: 10.1186/s13395-023-00324-0. Skelet Muscle. 2023. PMID: 37705115 Free PMC article.
-
Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons.Nat Commun. 2022 Sep 26;13(1):5628. doi: 10.1038/s41467-022-33184-1. Nat Commun. 2022. PMID: 36163250 Free PMC article.
-
Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair.Nat Commun. 2024 Aug 15;15(1):6808. doi: 10.1038/s41467-024-50628-y. Nat Commun. 2024. PMID: 39147780 Free PMC article.
-
Down-regulation of Sox11 is required for efficient osteogenic differentiation of adipose-derived stem cells.Mol Cells. 2014 Apr;37(4):337-44. doi: 10.14348/molcells.2014.0021. Epub 2014 Apr 7. Mol Cells. 2014. PMID: 24722414 Free PMC article.
-
Scorpion (Hottentotta tamulus) venom pre-exposure delays functional recovery in mice following peripheral nerve injury.PLoS One. 2025 Aug 19;20(8):e0330600. doi: 10.1371/journal.pone.0330600. eCollection 2025. PLoS One. 2025. PMID: 40828807 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

