Calcium-stores mediate adaptation in axon terminals of olfactory receptor neurons in Drosophila
- PMID: 22024464
- PMCID: PMC3226658
- DOI: 10.1186/1471-2202-12-105
Calcium-stores mediate adaptation in axon terminals of olfactory receptor neurons in Drosophila
Abstract
Background: In vertebrates and invertebrates, sensory neurons adapt to variable ambient conditions, such as the duration or repetition of a stimulus, a physiological mechanism considered as a simple form of non-associative learning and neuronal plasticity. Although various signaling pathways, as cAMP, cGMP, and the inositol 1,4,5-triphosphate receptor (InsP3R) play a role in adaptation, their precise mechanisms of action at the cellular level remain incompletely understood. Recently, in Drosophila, we reported that odor-induced Ca2+-response in axon terminals of olfactory receptor neurons (ORNs) is related to odor duration. In particular, a relatively long odor stimulus (such as 5 s) triggers the induction of a second component involving intracellular Ca2+-stores.
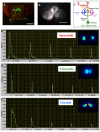
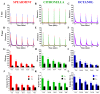
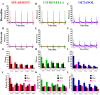
Results: We used a recently developed in-vivo bioluminescence imaging approach to quantify the odor-induced Ca2+-activity in the axon terminals of ORNs. Using either a genetic approach to target specific RNAs, or a pharmacological approach, we show that the second component, relying on the intracellular Ca2+-stores, is responsible for the adaptation to repetitive stimuli. In the antennal lobes (a region analogous to the vertebrate olfactory bulb) ORNs make synaptic contacts with second-order neurons, the projection neurons (PNs). These synapses are modulated by GABA, through either GABAergic local interneurons (LNs) and/or some GABAergic PNs. Application of GABAergic receptor antagonists, both GABAA or GABAB, abolishes the adaptation, while RNAi targeting the GABABR (a metabotropic receptor) within the ORNs, blocks the Ca2+-store dependent component, and consequently disrupts the adaptation. These results indicate that GABA exerts a feedback control. Finally, at the behavioral level, using an olfactory test, genetically impairing the GABABR or its signaling pathway specifically in the ORNs disrupts olfactory adapted behavior.
Conclusion: Taken together, our results indicate that a relatively long lasting form of adaptation occurs within the axon terminals of the ORNs in the antennal lobes, which depends on intracellular Ca2+-stores, attributable to a positive feedback through the GABAergic synapses.
Figures







Similar articles
-
Presynaptic Ca2+ stores contribute to odor-induced responses in Drosophila olfactory receptor neurons.J Exp Biol. 2010 Dec 15;213(Pt 24):4163-73. doi: 10.1242/jeb.046474. J Exp Biol. 2010. PMID: 21112997
-
Slow presynaptic mechanisms that mediate adaptation in the olfactory pathway of Drosophila.Elife. 2019 Jun 10;8:e43735. doi: 10.7554/eLife.43735. Elife. 2019. PMID: 31169499 Free PMC article.
-
Interaction between cAMP and intracellular Ca(2+)-signaling pathways during odor-perception and adaptation in Drosophila.Biochim Biophys Acta. 2016 Sep;1863(9):2156-74. doi: 10.1016/j.bbamcr.2016.05.014. Epub 2016 May 19. Biochim Biophys Acta. 2016. PMID: 27212269
-
Presynaptic inhibition of olfactory receptor neurons in crustaceans.Microsc Res Tech. 2002 Aug 15;58(4):365-75. doi: 10.1002/jemt.10144. Microsc Res Tech. 2002. PMID: 12214303 Review.
-
GABAergic mechanisms that shape the temporal response to odors in moth olfactory projection neurons.Ann N Y Acad Sci. 1998 Nov 30;855:475-81. doi: 10.1111/j.1749-6632.1998.tb10608.x. Ann N Y Acad Sci. 1998. PMID: 9929641 Review.
Cited by
-
Databases in SenseLab for the genomics, proteomics, and function of olfactory receptors.Methods Mol Biol. 2013;1003:3-22. doi: 10.1007/978-1-62703-377-0_1. Methods Mol Biol. 2013. PMID: 23585030 Free PMC article.
-
Time-Dependent Odorant Sensitivity Modulation in Insects.Insects. 2022 Apr 2;13(4):354. doi: 10.3390/insects13040354. Insects. 2022. PMID: 35447796 Free PMC article. Review.
-
Multiple sites of adaptation lead to contrast encoding in the Drosophila olfactory system.Physiol Rep. 2016 Apr;4(7):e12762. doi: 10.14814/phy2.12762. Physiol Rep. 2016. PMID: 27053295 Free PMC article.
-
Presynaptic GABA Receptors Mediate Temporal Contrast Enhancement in Drosophila Olfactory Sensory Neurons and Modulate Odor-Driven Behavioral Kinetics.eNeuro. 2016 Aug 23;3(4):ENEURO.0080-16.2016. doi: 10.1523/ENEURO.0080-16.2016. eCollection 2016 Jul-Aug. eNeuro. 2016. PMID: 27588305 Free PMC article.
-
Modulation of the NO-cGMP pathway has no effect on olfactory responses in the Drosophila antenna.Front Cell Neurosci. 2023 May 25;17:1180798. doi: 10.3389/fncel.2023.1180798. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37305438 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

