Cysteine cathepsins: from structure, function and regulation to new frontiers
- PMID: 22024571
- PMCID: PMC7105208
- DOI: 10.1016/j.bbapap.2011.10.002
Cysteine cathepsins: from structure, function and regulation to new frontiers
Abstract
It is more than 50 years since the lysosome was discovered. Since then its hydrolytic machinery, including proteases and other hydrolases, has been fairly well identified and characterized. Among these are the cysteine cathepsins, members of the family of papain-like cysteine proteases. They have unique reactive-site properties and an uneven tissue-specific expression pattern. In living organisms their activity is a delicate balance of expression, targeting, zymogen activation, inhibition by protein inhibitors and degradation. The specificity of their substrate binding sites, small-molecule inhibitor repertoire and crystal structures are providing new tools for research and development. Their unique reactive-site properties have made it possible to confine the targets simply by the use of appropriate reactive groups. The epoxysuccinyls still dominate the field, but now nitriles seem to be the most appropriate "warhead". The view of cysteine cathepsins as lysosomal proteases is changing as there is now clear evidence of their localization in other cellular compartments. Besides being involved in protein turnover, they build an important part of the endosomal antigen presentation. Together with the growing number of non-endosomal roles of cysteine cathepsins is growing also the knowledge of their involvement in diseases such as cancer and rheumatoid arthritis, among others. Finally, cysteine cathepsins are important regulators and signaling molecules of an unimaginable number of biological processes. The current challenge is to identify their endogenous substrates, in order to gain an insight into the mechanisms of substrate degradation and processing. In this review, some of the remarkable advances that have taken place in the past decade are presented. This article is part of a Special Issue entitled: Proteolysis 50 years after the discovery of lysosome.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
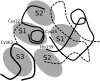


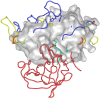
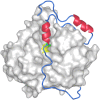
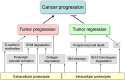
References
-
- De Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7:847–849. - PubMed
-
- De Duve C. Lysosomes revisited. Eur. J. Biochem. 1983;137:391–397. - PubMed
-
- De Duve C. Lysosomes, a new group of cytoplasmic particles. In: Hayashi T., editor. Subcellular Particles. The Ronald Press Co.; New York: 1959. pp. 128–159.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

